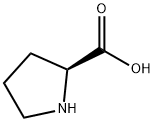
L-Proline synthesis
- Product Name:L-Proline
- CAS Number:147-85-3
- Molecular formula:C5H9NO2
- Molecular Weight:115.13
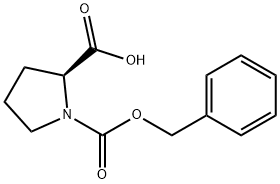
1148-11-4
453 suppliers
$6.00/10g

147-85-3
1058 suppliers
$6.00/25g
Yield:147-85-3 100%
Reaction Conditions:
with hydrogen;palladium on activated charcoal in ethyl acetate at 80; for 0.0833333 h;Microwave irradiation;
Steps:
A number of hydrogenation reactions were conducted using the present apparatus and method. Several of these reactions, along with their reaction conditions and yields, are listed below. Unless otherwise noted, each reaction proceeded with a stoichiometric amount of hydrogen with respect to the reactant to be hydrogenated and 1% catalyst loading.As used in the drawings, the abbreviation Pd/C refers to a palladium metal catalyst on a carbon support. The abbreviation EtOH refers to ethyl alcohol and the abbreviation EtOAc refers to ethyl acetate. The abbreviation μλ refers to the application of microwaves. Simultaneous cooling was carried out using the PowerMAX single mode capacity microwave instrument available from the assignee herein, CEM Corporation of Matthews, N.C., USA.
References:
US2007/209924,2007,A1 Location in patent:Page/Page column 7
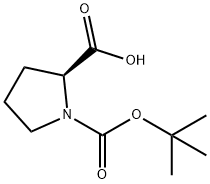
15761-39-4
605 suppliers
$5.00/10g

147-85-3
1058 suppliers
$6.00/25g
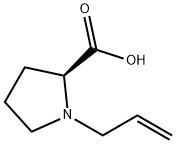
610299-77-9
13 suppliers
$250.88/1g

147-85-3
1058 suppliers
$6.00/25g