L-(-)-SORBOSE synthesis
- Product Name:L-(-)-SORBOSE
- CAS Number:87-79-6
- Molecular formula:C6H12O6
- Molecular Weight:180.16
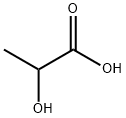
57-48-7
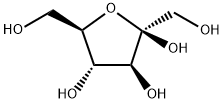
849585-22-4
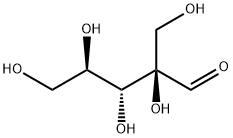
4573-78-8

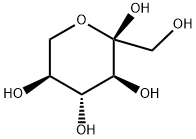
87-79-6
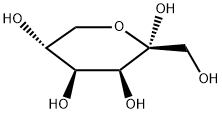
87-81-0
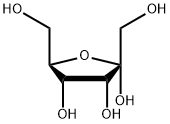
551-68-8
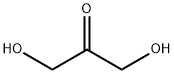
96-26-4
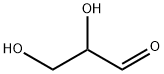
56-82-6
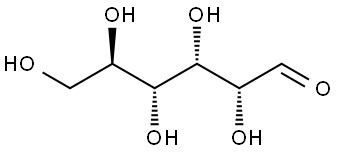
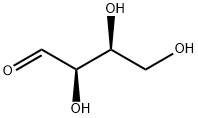
The target compounds were synthesized from D-fructose by the following steps: 2-hydroxypropanoic acid, D-witch hazel sugar, (3S,4R,5S)-1,3,4,5,6-pentahydroxyhexan-2-one, (3S,4S,5R)-1,3,4,5,6-pentahydroxyhexan-2-one, (3R,4R,5R)-1,3,4,5,6-pentahydroxyhexan-2-one, 1 ,3-dihydroxyacetone and DL-glyceraldehyde. This was done as follows: the D-fructose was reacted with MoO3 in water at 100 °C for 4 h. After completion of the reaction, the fructose-containing grades were separated by appropriate methods. Analysis using 1H NMR spectroscopy (shown in Fig. 8) confirmed the presence of sorbose in the separated grades.
Yield:-
Reaction Conditions:
with silica zeolite beta containing Lewis acidic framework Ti4+ in methanol at 99.84; for 2 h;Kinetics;Sealed tube;Reagent/catalyst;
Steps:
2.2 Kinetic Studies of Glucose Reactions with Ti-Beta
Reactions with D-glucose (Sigma-Aldrich, ≧99%) were conducted in 10 mL thick-walled glass batch reactors (VWR), with temperature control via an oil bath located on a digital stirring hotplate (Fisher Scientific). Typical reactions with D-glucose were carried out at a 1:50 metal:glucose molar ratio and involved contacting 4 g of a 1% (w/w) glucose solution in water or in methanol with the catalytic solids in a stirred glass reactor sealed with a crimp top (PTFE/silicone septum, Agilent). Kinetic studies using isotopically-labeled glucose were performed using 1% (w/w) solutions of D-glucose-D2 (Cambridge Isotope Laboratories, ≧98%) or of D-glucose-D2-1,2,3,4,5,6,6 (Cambridge Isotope Laboratories, ≧98%) in methanol. [0080] Reactors were placed in the oil bath and small aliquots (50-100 microliters) were extracted at various time intervals via syringe (Hamilton), filtered through a 0.2 micron PTFE filter (National Scientific), and mixed with 1% (w/w) aqueous D-mannitol (Sigma-Aldrich, ≧98%) solutions used as an internal standard for quantification. The composition of reaction aliquots was determined after separation of compounds in an Agilent 1200 high performance liquid chromatograph (HPLC) equipped with an evaporative light scattering (ELS) detector (Agilent 380 LC). Glucose, sorbose, mannose, fructose, and mannitol fractions were separated using a Hi-Plex Ca column (7.7×300 mm, 8 micron particle size, Agilent) held at 353 K, with either ultrapure water (0.010 mL s-1 flow rate) or a 30/70 (v/v) mixture of acetonitrile/water (0.013 mL s-1 flow rate) as the mobile phase.
References:
CALIFORNIA INSTITUTE OF TECHNOLOGY;DAVIS, MARK E.;GOUNDER, RAJAMANI US2014/309415, 2014, A1 Location in patent:Paragraph 0079; 0080

57-48-7
590 suppliers
$12.00/250G

849585-22-4
1 suppliers
inquiry

4573-78-8
48 suppliers
$60.00/100 mg

87-79-6
276 suppliers
$34.00/10g

87-81-0
383 suppliers
$12.50/5g

551-68-8
223 suppliers
$28.00/250G

96-26-4
495 suppliers
$9.00/10g

56-82-6
172 suppliers
$68.90/500mg
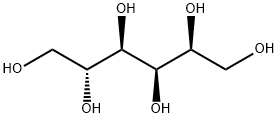
50-70-4
797 suppliers
$5.00/10g

87-79-6
276 suppliers
$34.00/10g