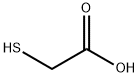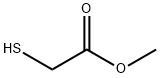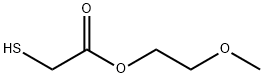
Mercaptoacetic acid 2-methoxyethyl ester synthesis
- Product Name:Mercaptoacetic acid 2-methoxyethyl ester
- CAS Number:19788-48-8
- Molecular formula:C5H10O3S
- Molecular Weight:150.2
Yield:19788-48-8 84%
Reaction Conditions:
with sulfuric acid in toluene;Reflux;
Steps:
1.xix
xix) Synthesis of 2-methoxyethyl 2-((4-oxo-4-(2,6,6-trimethylcyclohex-3-en-l- yl)butan-2-yl)thio)acetate 2-Methoxyethanol (3.66 g, 48.1 mmol), thioglycolic acid (5.00 ml, 72.2 mmol) and dry toluene (50 ml) were stirred and a catalytic amount of sulfuric acid (one drop) was added. The solution was refluxed overnight. After cooling to room temperature, the solvent was evaporated to give a colorless oil. The oil was taken up in dichloromethane (50 ml) and washed with water (2 twice 20 ml) and brine (40 ml). The organic phase was then dried over sodium sulfate, filtered, and concentrated to give 2-methoxyethyl 2-mercaptoacetate (6.07 g, 84% yield) as a colorless oil. Delta-damascone (3.00 g, 15.6 mmol), DBU (0.24 ml, 1.6 mmol) and tetrahydrofuran (THF, 20 ml) were heated to 45°C with stirring. A solution of 2-methoxyethyl 2- mercaptoacetate (15.6 mmol) in THF (10 ml) was added dropwise. The mixture was stirred for 12 h, cooled to room temperature and then concentrated to give a purple oil. The oil was taken up in dichloromethane (50 ml) and successively washed with aqueous HCl (5%, 2 X 20 ml), water (2 X 20 ml), and brine (40 ml). The organic phase was dried over sodium sulfate, filtered and concentrated to give a yellow oil with 61% yield. 13C-NMR: 19.9 (q), 20.7 (q), 21.1/21.3 (q), 29.8 (q), 31.6/31.8 (d), 32.8/33.0 (t), 33.1/33.2 (s), 35.3 (d), 41.7 (t), 54.7/54.8 (t), 59.0 (q), 62.8/62.9 (d), 64.3 (t), 70.3 (t), 124.1/124.3 (d), 131.7/131.8 (d), 170.6 (s), 211.8/212.0 (s). 'H-NMR: 0.86-0.92 (m, 3H); 0.93-1.01 (m, 6H); 1.29-1.36 (m, 3H); 1.65-1.75 (m, 1H); 1.91-2.02 (m, 1H); 2.18-2.25 (m, 1H); 2.45-2.55 (m, 1H); 2.72-2.78 (m, 1H); 2.50- 2.60 and 2.92-3.00 (2 m, 1H); 3.27-3.38 (m, 2H); 3.39 (s, 3H); 3.40-3.50 (m, 1H); 3.58-3.65 (m, 2H); 4.26-4.32 (m, 2 H); 5.41-5.48 (m, 1H); 5.50-5.58 (m, 1H).
References:
WO2013/139766,2013,A1 Location in patent:Page/Page column 25-26