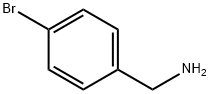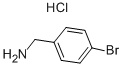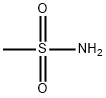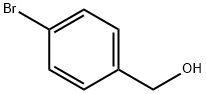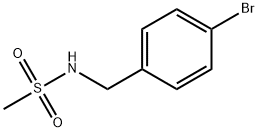
Methanesulfonamide, N-[(4-bromophenyl)methyl]- synthesis
- Product Name:Methanesulfonamide, N-[(4-bromophenyl)methyl]-
- CAS Number:183128-59-8
- Molecular formula:C8H10BrNO2S
- Molecular Weight:264.14
Yield:183128-59-8 97%
Reaction Conditions:
with triethylamine in dichloromethane at 20;Cooling with ice;Inert atmosphere;
Steps:
25
A solution of 4-bromobenzylamine (2.50 g, 13.5 mmol) and triethylamine (14.9 mmol, 1.5 g, 2.1 ml) in dichloromethane (50 ml) was cooled in an ice bath with stirring under argon. Methanesulfonyl chloride (1.55 g, 13.7 mmol, 1.05 ml) was added dropwise and the resulting mix was allowed to warm up to room temperature and stirred for 1 hour. The reaction mix was washed 3 times with water (20 ml) and the organic layer deied over sodium sulphate and reduced to minimum volume under reduced pressure to give the title compound as a colourless solid (3.45 g, 97%).1H-NMR (400 MHz, CDCl3) δ: 7.51 (2H, m), 7.25 (2H, m), 4.67 (1H, m), 4.29 (2H, d, J=6 Hz), 2.90 (3H, s); LC/MS Retention time 2.39 mins/(ES-) 262 & 264 (M-H, C8H10BrNO2S requires 263 & 265).
References:
US2010/137276,2010,A1 Location in patent:Page/Page column 22
1122-91-4
702 suppliers
$9.00/10g
![Methanesulfonamide, N-[(4-bromophenyl)methyl]-](/CAS/20210111/GIF/183128-59-8.gif)
183128-59-8
2 suppliers
inquiry