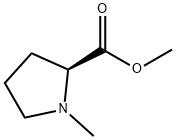
Methyl 1-methylpyrrolidine-2-carboxylate synthesis
- Product Name:Methyl 1-methylpyrrolidine-2-carboxylate
- CAS Number:933-94-8
- Molecular formula:C7H13NO2
- Molecular Weight:143.18
Yield:-
Reaction Conditions:
with formaldehyd;sodium borohydrid;sulfuric acid in methanol;water;
Steps:
6.a 6a.
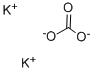
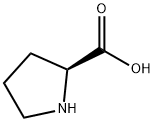
6a. N-Methyl-proline Methyl Ester Methanol (2.2 L, 68.66 mol) was stirred under nitrogen and cooled to 0° C. Sulfuric acid (97%, 0.179 L, 3.26 mol) was added with vigorous stirring at a rate such that the internal temperature of the reactor did not rise above 10° C. L-Proline (0.5 kg, 4.34 mol) was added to the methanol, and the reaction was refluxed for 18 hr. The reaction mixture was cooled to 0° C., and with vigorous stirring a solution of K2 CO3 (0.217 kg, 1.57 mol) in 0.363 L of water was slowly added to the mixture, until the pH was between pH 7 and 8. The neutralized solution was again cooled to 0° C., and aqueous formaldehyde solution (36%, 0.543 L) was added. After stirring for 15 min, 0.082 kg (2.17 mol) of powdered sodium borohydride was added in portions while maintaining the temperature between -5° and +5° C. The suspension was stirred for 3 hr, then filtered, and the filter cake was washed with toluene. The combined organic solvents were diluted 1:1 with water (2.6 L) and filtered. The organic phase was separated, and the aqueous layer was extracted at 0° C. with 5*0.5 L portions of toluene. The toluene extracts were combined, dried over sodium sulfate and filtered. The volume of the solution was reduced by half by distillation at 60° C. and 90 mbar pressure. The solution was taken to the next step without further purification.
References:
US5424444,1995,A
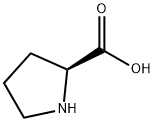
609-36-9
391 suppliers
$5.00/5g

933-94-8
10 suppliers
inquiry