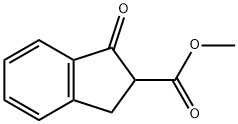
Methyl 1-oxo-2,3-dihydro-1H-indene-2-carboxylate synthesis
- Product Name:Methyl 1-oxo-2,3-dihydro-1H-indene-2-carboxylate
- CAS Number:22955-77-7
- Molecular formula:C11H10O3
- Molecular Weight:190.2
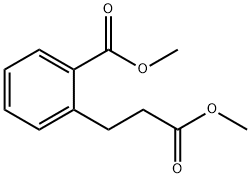
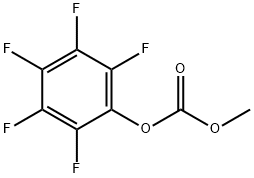
25040-17-9

22955-77-7
Methyl 2-[3-(methoxy)-3-oxopropyl]benzoate (3.77 g, 16.98 mmol) was dissolved in anhydrous THF (75 mL) under argon protection. Under stirring, sodium hydride (2.04 g, 60% oil suspension, 50.9 mmol) was slowly added over 2 min. Subsequently, the reaction mixture was slowly heated to reflux. After 1 hour of reflux reaction, formation of a thick paste was observed. The reaction mixture was cooled to room temperature and quenched by adding water (1 mL) dropwise over 5 min. Next, the reaction mixture was acidified with 5 M hydrochloric acid (20 mL) and extracted twice with ethyl acetate. The organic phases were combined, dried with anhydrous magnesium sulfate, and subsequently concentrated under reduced pressure to give an oil. The oily substance was purified by silica gel column chromatography (70 g) using a 0-50% ethyl acetate/pentane gradient elution to afford methyl 1-oxo-2,3-dihydro-1H-indene-2-carboxylate as a yellow oil (2.14 g, 66% yield), which solidified to a solid on standing. The product was characterized by 1H-NMR (400 MHz, CDCl3): δ 7.79 (1H, d, J = 7.6 Hz), 7.63 (1H, dd, J = 7.6 and 7.6 Hz), 7.50 (1H, d, J = 7.6 Hz), 7.42 (1H, dd, J = 7.6 and 7.6 Hz), 3.80 (3H, s), 3.75 ( 1H, m), 3.60 (1H, m), 3.40 (1H, m).
Yield: 99%
Reaction Conditions:
with sodium hydride in tetrahydrofuran at 20; for 2 h;Inert atmosphere;
Steps:
4.1 Step 1 Preparation of Intermediate 4-1
Dimethyl carbonate (17 g, 189 mmol) and THF (80 mL) were added to a 250 mL three-necked flask, and NaH (60% w/w, 3.18 g, 79.4 mmol) was added under stirring at room temperature and protected by nitrogen replacement. A solution of 1-indanone (5 g, 37.8 mmol) in THF (40 mL) was added dropwise to the reaction solution with a dropping funnel. After the addition, the temperature was raised to reflux for 2 hours, and TLC showed that the reaction was complete. Pour the reaction solution into a mixture of 1M HCl and ice, extract three times with EA (100 mL), combine the EA layers, dry, and spin dry to obtain black oil 4-1 (7.11 g, yield: 99%), which is used directly for the next One step response.
References:
CN112341429, 2021, A Location in patent:Paragraph 0124-0128

25040-17-9
1 suppliers
inquiry

22955-77-7
43 suppliers
inquiry
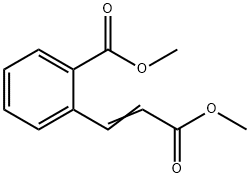
63600-27-1
0 suppliers
inquiry

22955-77-7
43 suppliers
inquiry

124-38-9
134 suppliers
$175.00/23402
83-33-0
571 suppliers
$6.00/5g

74-88-4
356 suppliers
$15.00/10g

22955-77-7
43 suppliers
inquiry