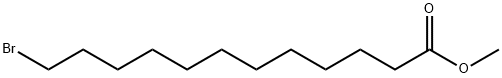
methyl 12-bromododecanoate synthesis
- Product Name:methyl 12-bromododecanoate
- CAS Number:26825-95-6
- Molecular formula:C13H25BrO2
- Molecular Weight:293.24

67-56-1
73367-80-3

26825-95-6
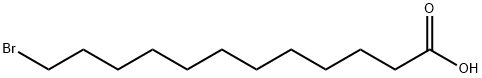
Under argon protection, 12-bromododecanoic acid (6) (1.0 g, 3.59 mmol) was dissolved in anhydrous methanol (10 mL) containing concentrated sulfuric acid (200 μL) and the reaction was heated to reflux for 15 hours. After completion of the reaction, methanol was removed by distillation under reduced pressure. The residue was diluted with ethyl acetate (100 mL), washed sequentially with saturated sodium bicarbonate solution (3×20 mL) and brine (2×20 mL), and the organic phase was dried with anhydrous magnesium sulfate. The solvent was removed by concentration under reduced pressure to obtain the crude product. Purification by silica gel column chromatography using petroleum ether/ethyl acetate (97.5:2.5) as eluent afforded the target product methyl 12-bromododecanoate (7) (974 mg, 3.32 mmol, 93% yield) as a colorless oil. The product was analyzed by thin-layer chromatography (TLC, Rf 0.45, petroleum ether/ethyl acetate, 9:1), infrared spectroscopy (IR), nuclear magnetic resonance hydrogen spectroscopy (1H NMR), nuclear magnetic resonance carbon spectroscopy (13C NMR) and high-resolution mass spectrometry (HRMS).
Yield: 2.65 g (90%)
Reaction Conditions:
with triphenylphosphine in toluene
Steps:
21.1 Example 21
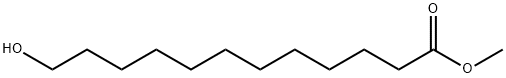
(1) Methyl 12-hydroxydodecanoate (2.30 g, 10.0 mmol), triphenylphosphine (3.93 g, 1.5 eq), carbon tetrabromide (4.97 g, 1.5 eq) were stirred in toluene (10 ml) at room temperature for 3 hr. The reaction solution was filtered, followed by concentration to dryness. The residue was purified by column chromatography on silica gel (toluene-ethyl acetate (99: 1)) to give 2.65 g (90%) of methyl 12-bromododecanoate. 1H-NMR (CDCl3) δ: 1.20-1.35 (12H, m), 1.39-1.43 (2H, m), 1.56-1.63 (2H, m), 1.82-1.88 (2H, m), 2.30 (2H, t, J = 7.6 Hz), 3.41 (2H, t, J = 7.6 Hz), 3.73 (3H, s) IR (KBr): 1742 cm-1EI-MS m/z: 293 (M+)
References:
Drug Delivery System Institute, Ltd.;MEIJI SEIKA KAISHA LTD.;Asahi Kasei Kogyo Kabushiki Kaisha EP953357, 1999, A1
71655-36-2
10 suppliers
inquiry

26825-95-6
39 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

26825-95-6
39 suppliers
inquiry
947-05-7
19 suppliers
$48.79/1g

26825-95-6
39 suppliers
inquiry