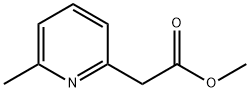
Methyl 2-(6-Methylpyridin-2-yl)acetate synthesis
- Product Name:Methyl 2-(6-Methylpyridin-2-yl)acetate
- CAS Number:58532-56-2
- Molecular formula:C9H11NO2
- Molecular Weight:165.19
Yield:58532-56-2 35%
Reaction Conditions:
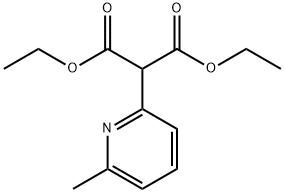
Stage #1: 2-(6-methylpyridin-2-yl)malonic acid diethyl esterwith potassium hydroxide;ethanol for 1 h;Heating / reflux;
Stage #2: with hydrogenchloride;water at 0;
Stage #3: diazomethyl-trimethyl-silanewith methanol in hexane; for 5 h;
Steps:
Intermediate 90: methyl (6-methyl-2-pyridinyl)acetate; 668 mg of potassium hydroxide (1 1.9 mmole) were added to a solution of 300 mg of intermediate 89 (1.19 mmole) in 40 ml. of EtOH. The mixture was heated at reflux for 1 h, then it was allowed to cool to RT and concentrated in vacuo. The crude was passed trough a SCX column, the collected fractions were concentrated and the resulted potassium salt was dissolved in 5 ml. of water, cooled to O0C and treated with HCI 1 N. The solvent was removed in vacuo to give 160 mg of a crude that was dissolved in 8 ml_ of dry MeOH, then 850 μl_ of (trimethysilyl)diazomethane 2M in hexans (1.7 mmole, Aldrich) were added dropwise. After stirring for 3 h, further (trimethysilyl)diazomethane (1.7 mmole) was added and the reaction mixture was stirred for additional 2 h. Then it was quenched with acetic acid (glacial, Aldrich) and the solvents were evaporated in vacuo. The reaction was repeated using 360 mg of intermediate 20 and the combined crude were purified by flash chromatography (CH/AcOEt from 9:1 to 8:2) to give the title compound (160 mg, 35%). 1H-NMR (400 MHz, CDCI3): δ 2.55 (3H, s), 3.8 (3H, s), 3.9 (2H, s), 7.1 (2H, dd), 7.55 (1 H, t); m/z (ES): 166 [M+H]+.
References:
WO2008/148853,2008,A1 Location in patent:Page/Page column 114
108-48-5
490 suppliers
$9.90/190μL

58532-56-2
26 suppliers
inquiry