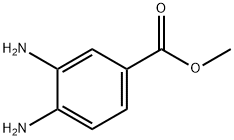
Methyl 3,4-diaminobenzoate synthesis
- Product Name:Methyl 3,4-diaminobenzoate
- CAS Number:36692-49-6
- Molecular formula:C8H10N2O2
- Molecular Weight:166.18

67-56-1
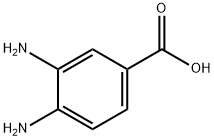
619-05-6

36692-49-6
Thionyl chloride (0.72 mL, 9.80 mmol) was slowly added dropwise to a solution of 3,4-diaminobenzoic acid (1.00 g, 6.58 mmol) in methanol (15.0 mL) for a controlled time of 10 minutes. The reaction mixture was stirred at room temperature for 4 hours before the solvent was removed by evaporation under reduced pressure. The residue was partitioned between water and ethyl acetate (EtOAc) to separate the organic layer. The organic layer was diluted with ethyl acetate and washed sequentially with saturated aqueous sodium bicarbonate, saturated brine and water and then dried with anhydrous magnesium sulfate. The desiccant was removed by filtration and the solvent was evaporated under reduced pressure to afford methyl 3,4-diaminobenzoate (993 mg, 95.0% yield) as a brown solid. The product characterization data were as follows: 1H NMR (CDCl3, 500 MHz) δ 7.49 (d, 1H, J = 7.01 Hz, Ar-H), 7.47 (s, 1H, Ar-H), 6.68 (d, 1H, J = 7.01 Hz, Ar-H), 3.87 (s, 3H, -COOCH3), 3.80 (br s, 2H, -NH2 ), 3.35 (br s, 2H, -NH2); 13C NMR (CDCl3, 125 MHz) δ 166.56, 140.89, 138.16, 120.87, 118.23, 118.16, 52.11; LCMS m/z calculated value (MNa)+ 189.06, measured value 189.00.

67-56-1
790 suppliers
$9.00/25ml

619-05-6
306 suppliers
$19.00/25g

36692-49-6
256 suppliers
$11.00/5g
Yield:36692-49-6 98.1%
Reaction Conditions:
with sulfuric acid at 90; for 12 h;Temperature;
Steps:
1.1; 2.1; 3.1; 4.1; 5.1; 6.1 (1)Synthesis of methyl 3,4-diaminobenzoate (2)
Take a 100 ml single-mouth bottle, add 3,4-diaminobenzoic acid (1) 3.04 g (0.02 mol), and add 50 mL of methanol.Stir and dissolve, then add 19.6 g (0.2 mol) of concentrated sulfuric acid.Stir at 90 ° C for 12 hours. After the reaction is completed,Methanol is distilled off under reduced pressure, and the residue is poured into ice water.Drying by suction filtration to obtain methyl 3,4-diaminobenzoate (2)3.26g,The yield was 98.1%.
References:
CN108997229,2018,A Location in patent:Paragraph 0015; 0025; 0028; 0031; 0034; 0037
99512-09-1
52 suppliers
inquiry

36692-49-6
256 suppliers
$11.00/5g
3987-92-6
136 suppliers
$10.00/1g

36692-49-6
256 suppliers
$11.00/5g

619-05-6
306 suppliers
$19.00/25g

36692-49-6
256 suppliers
$11.00/5g
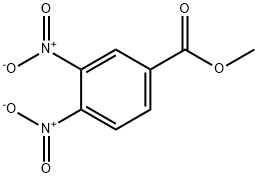
22907-68-2
11 suppliers
inquiry

36692-49-6
256 suppliers
$11.00/5g