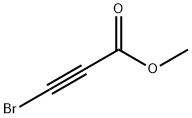
Methyl-3-bromopropiolate synthesis
- Product Name:Methyl-3-bromopropiolate
- CAS Number:23680-40-2
- Molecular formula:C4H3BrO2
- Molecular Weight:162.97
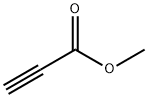
922-67-8

23680-40-2
The general procedure for the synthesis of methyl 3-bromopropiolate from methyl propiolate: methyl propiolate (60 g, 713.6 mmol) was dissolved in acetone (2 L), followed by the sequential addition of N-bromosuccinimide (147.22 g, 827.13 mmol) and silver nitrate (12.12 g, 71.37 mmol). A slight exothermic phenomenon was observed during the reaction and the reaction temperature was increased from 21°C to 32°C. The reaction mixture was stirred at room temperature overnight. Upon completion of the reaction, the resulting gray suspension was evaporated by vacuum to remove the solvent, pentane (100 mL) was added and filtered through Celite, and the filter cake was washed with additional pentane. The process was repeated twice and all filtrates were combined and vacuum evaporated to give 113 g of methyl 3-bromopropiolate in 98% yield, which contained about 10% unreacted material. The product was characterized by 1H NMR (400 MHz, CDCl3) with a chemical shift δ of 3.78 (s, 3H).
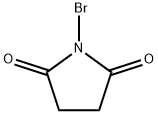
128-08-5
894 suppliers
$5.00/5 g

922-67-8
355 suppliers
$6.00/1g

23680-40-2
64 suppliers
$49.00/100mg
Yield: 88%
Reaction Conditions:
with silver nitrate in acetone at 20; for 6 h;
Steps:
Preparation of tert-butyl (1S,2R,4R)-2-amino-7-azabicyclo[2.2.1]heptane-7-carboxylate
Methyl propiolate (52 ml, 0.583 mol) is combined with recrystallized N- bromo-succinimide (120 g, 0.674 mol) in 1,700 ml acetone under nitrogen. The solution is treated with silver nitrate (9.9 g, 0.0583 mol) neat in a single lot and the reaction is stirred 6 h at RT. The acetone is removed under reduced pressure (25C, bath temperature) to provide a gray slurry. The slurry is washed with 2 x 200 ml hexane, the gray solid is removed by filtration, and the filtrate is concentrated in vacuo to provide 95 g of a pale yellow oily residue. The crude material is distilled via short path under reduced pressure (65C, about 25 mm Hg) into a dry ICE/ACETONE cooled receiver to give 83.7 g (88%) of methyl-3-bromo-propiolate as a pale yellow oil. Anal. calc'd for C4H3BRO2 : C, 29.48 ; H, 1.86. Found: C, 29.09 ; H, 1.97.
References:
PHARMACIA & UPJOHN COMPANY WO2004/39815, 2004, A2 Location in patent:Page 44

922-67-8
355 suppliers
$6.00/1g

23680-40-2
64 suppliers
$49.00/100mg

67-56-1
790 suppliers
$7.29/5ml-f
16900-53-1
15 suppliers
inquiry

23680-40-2
64 suppliers
$49.00/100mg