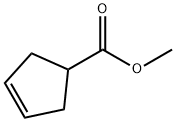
Methyl 3-cyclopentenecarboxylate synthesis
- Product Name:Methyl 3-cyclopentenecarboxylate
- CAS Number:58101-60-3
- Molecular formula:C7H10O2
- Molecular Weight:126.15

67-56-1
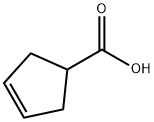
7686-77-3

58101-60-3
At 0 °C and under nitrogen protection, 3-cyclopentenecarboxylic acid (10 g, 89 mmol) was dissolved in dichloromethane (100 mL), followed by the addition of 1,1'-carbonyl diimidazole (43 g, 267 mmol). The reaction mixture was stirred at room temperature for 4 hours, after which methanol (100 mL) was added. Stirring of the reaction mixture was continued overnight. The solvent was removed by low temperature evaporation to afford methyl 3-cyclopentene-1-carboxylate (11.2 g, 99% yield).
Yield:58101-60-3 96%
Reaction Conditions:
with potassium carbonate in DMF (N,N-dimethyl-formamide) at 20; for 16 h;
Steps:
2.A Step A
To a solution of 3-cyclopentene-1-carboxylic acid (Org. Synth. 75, PL95-200, 1998) (31.5 g, 281 mmol) in anhydrous N, N-DIMETHYLFORMAMIDE (300 mL), under an atmosphere of nitrogen, was added potassium carbonate (97 g, 703 mmol), and iodomethane (35 mL, 563 mmol). The resulting mixture was stirred at room temperature for 16 hours, then poured into water (1 litre), and extracted with diethyl ether (3 x 400 mL). The combined diethyl ether layers were washed with water (3 x 500 mL), saturated NACL (200 mL), dried over MGS04, filtered and concentrated I71 VACUO, to give 34 g (96 %) of crude product. H NMR (CDC13, 500 MHz) : 8 5.64 (s, 2H), 3.68 (s, 3H), 3.11 (quintet, J = 8.5 Hz, 1H), 2.63 (d, J = 8.3 Hz, 4 H).
References:
MERCK & CO., INC. WO2004/41777, 2004, A2 Location in patent:Page 41-42

67-56-1
790 suppliers
$7.29/5ml-f

7686-77-3
266 suppliers
$6.00/1g

58101-60-3
165 suppliers
$6.00/1g
84646-68-4
52 suppliers
$45.00/50mg

58101-60-3
165 suppliers
$6.00/1g
54385-33-0
16 suppliers
$1540.00/1g

58101-60-3
165 suppliers
$6.00/1g

67-56-1
790 suppliers
$7.29/5ml-f
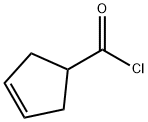
3744-80-7
6 suppliers
$141.38/250mgs:

58101-60-3
165 suppliers
$6.00/1g
