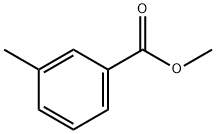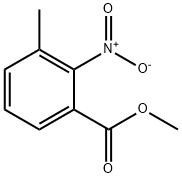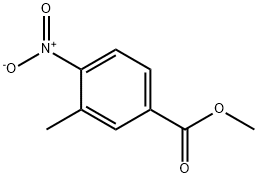
Methyl 3-methyl-4-nitrobenzoate synthesis
- Product Name:Methyl 3-methyl-4-nitrobenzoate
- CAS Number:24078-21-5
- Molecular formula:C9H9NO4
- Molecular Weight:195.17

67-56-1
3113-71-1

24078-21-5
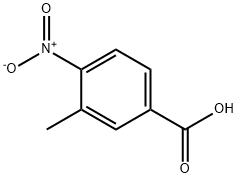
Step 1. Synthesis of methyl 3-methyl-4-nitrobenzoate 3-Methyl-4-nitrobenzoic acid (30 g, 165.61 mmol) was dissolved in methanol (300 mL), stirred at 0°C and thionyl chloride (25 mL) was added slowly and dropwise. The reaction mixture was warmed to 80°C and stirring was continued for 1 hour. Upon completion of the reaction, the mixture was concentrated under reduced pressure to give the crude product. The crude product was dissolved in petroleum ether (100 mL) and filtered to give methyl 3-methyl-4-nitrobenzoate as a white solid (31 g, 96% yield). Product Characterization: LC/MS (ES, m/z): [M + H]+ 196.01 1H-NMR (300MHz, CD3Cl) δ 7.99-8.04 (m, 3H), 3.98 (s, 3H), 2.64 (s, 3H)

67-56-1
790 suppliers
$7.29/5ml-f

3113-71-1
485 suppliers
$6.00/25g

24078-21-5
248 suppliers
$6.00/5g
Yield:24078-21-5 96%
Reaction Conditions:
with thionyl chloride at 0 - 80; for 1 h;
Steps:
40.1
Step 1. Methyl 3-methyl-4-nitrobenzoate To a solution of 3-methyl-4-nitrobenzoic acid (30 g, 165.61 mmol) in methanol (300 mL), was added thionyl chloride (25 mL) dropwise with stirring at 0° C. After stirring for 1 h at 80° C., the resulting mixture was concentrated under vacuum to give residue, which was dissolved in petroleum ether (100 mL) and filtrated to give methyl 3-methyl-4-nitrobenzoate as a white solid (31 g, 96%). LC/MS (ES, m/z): [M+H]+ 196.0 1H-NMR (300 MHz, CD3Cl) δ 7.99-8.04 (m, 3H), 3.98 (s, 3H), 2.64 (s, 3H)
References:
BIOENERGENIX US2012/277224, 2012, A1 Location in patent:Page/Page column 33
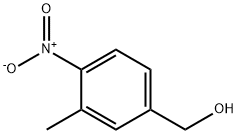
80866-75-7
91 suppliers
$23.00/1g
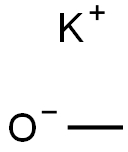
865-33-8
174 suppliers
$12.50/100G

24078-21-5
248 suppliers
$6.00/5g

3113-71-1
485 suppliers
$6.00/25g

24078-21-5
248 suppliers
$6.00/5g
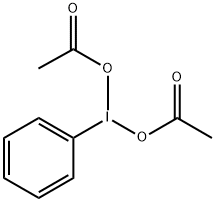
3240-34-4
612 suppliers
$7.00/10g

3113-71-1
485 suppliers
$6.00/25g

24078-21-5
248 suppliers
$6.00/5g