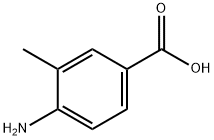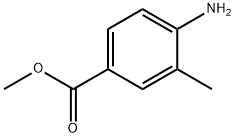
Methyl 4-amino-3-methylbenzoate synthesis
- Product Name:Methyl 4-amino-3-methylbenzoate
- CAS Number:18595-14-7
- Molecular formula:C9H11NO2
- Molecular Weight:165.19
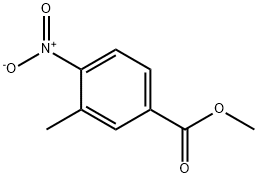
24078-21-5

18595-14-7
The general procedure for the synthesis of methyl 4-amino-3-methylbenzoate from methyl 3-methyl-4-nitrobenzoate was as follows: methyl 3-methyl-4-nitrobenzoate (50 g, 256.2 mmol) was dissolved in methanol (1.5 L), and palladium carbon catalyst (2.5 g) was added. Hydrogen (H2) was passed into the reaction mixture at room temperature and the reaction was continuously stirred overnight. Upon completion of the reaction, the solid catalyst was removed by filtration and the filtrate was concentrated under reduced pressure to afford the light yellow solid product methyl 4-amino-3-methylbenzoate (38 g, 90% yield). The product was analyzed by LCMS (electrospray ionization, positive ion mode) with a mass-to-charge ratio (m/z) of 166.1, corresponding to the [M + H]+ ion peak.
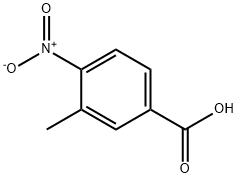
3113-71-1
483 suppliers
$6.00/25g

18595-14-7
300 suppliers
$8.00/5g
Yield:18595-14-7 99%
Reaction Conditions:
with H2;palladium-carbon in methanol;
Steps:
17.a Example 17
(a) Methyl 4-amino-3-methylbenzoate. To a solution of 3-methyl-4-nitrobenzoic acid (6.50 g, 33.3 mmol) in 100 mL of MeOH was added 700 mg of 5% Pd/C. The mixture was hydrogenated at 48 psi H2 for 24 hours, the catalyst was removed by suction filtration, and the filtrate was concentrated under reduced pressure. The title compound was obtained as a white solid (99%). 1H NMR (CDCl3) δ: 2.19 (s, 3H), 3.85 (s, 3H), 4.20 (br s, 2H), 6.65 (d, 2H, J = 8.1Hz), 7.72 (d, 2H, J = 8.1Hz), 7.76 (s, 1H). Anal. (C9H11NO2) C, H, N.
References:
EP1109560,2003,B1

24078-21-5
250 suppliers
$6.00/5g

18595-14-7
300 suppliers
$8.00/5g
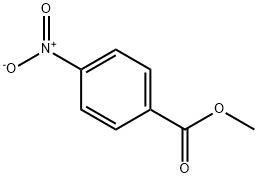
619-50-1
184 suppliers
$17.00/25g
676-58-4
294 suppliers
$15.00/25ml
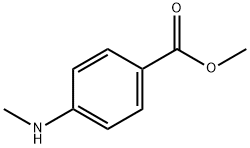
18358-63-9
118 suppliers
$15.00/1g

18595-14-7
300 suppliers
$8.00/5g