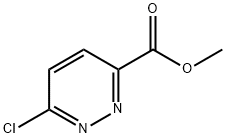
METHYL 6-CHLOROPYRIDAZINE-3-CARBOXYLATE synthesis
- Product Name:METHYL 6-CHLOROPYRIDAZINE-3-CARBOXYLATE
- CAS Number:65202-50-8
- Molecular formula:C6H5ClN2O2
- Molecular Weight:172.57
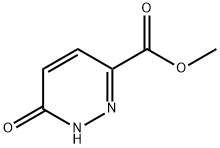
63001-30-9

65202-50-8
B. Methyl 6-hydroxypyridazine-3-carboxylate prepared as described above was mixed with phosphorochloridic acid, slowly heated to reflux temperature and the reaction was maintained for 2.5 hours. Upon completion of the reaction, the mixture was cooled to room temperature and the excess phosphoryl chloride was subsequently evaporated under reduced pressure. The residue was carefully decanted into ice water and the precipitated solid was collected by filtration. The precipitate was washed sequentially with saturated sodium bicarbonate solution and deionized water and finally dried in a vacuum drying oven to afford the target product, methyl 6-chloropyridazine-3-carboxylate, as a yellow solid (4.359 g, 79% yield).

67-56-1
790 suppliers
$7.29/5ml-f
5096-73-1
187 suppliers
$15.00/1g

65202-50-8
179 suppliers
$21.21/1gm:
Yield:65202-50-8 88.2%
Reaction Conditions:
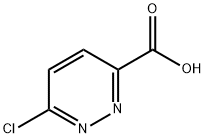
Stage #1:6-chloro-pyridazine-3-carboxylic acid with oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane at 0 - 20; for 1 h;
Stage #2:methanol in dichloromethane at 20; for 1 h;
Steps:
1.11; 1.13
Oxalyl chloride (1 equiv.) was added dropwise to a solution of 6-chloro-3- pyridazinecarboxylic acid (1.000 g, 6.308 mmol) in dry DCM (30 mL) and dry DMF (1 drop) at 0 °C and the mixture stirred at this temperature for 1 h. The solvent was removed in vacuo and the residue redissolved in dry DCM. MeOH (1 equiv.) were added and the mixture stirred at rt for a further 1 h. The mixture was quenched with H2O (20 mL) and extracted with DCM (2 x 20 mL). The combined organic layers were then dried over MgSO4and concentrated to give methyl 6-chloropyridazine-3-carboxylate as a white solid. (0.960 g, 88.2% yield). NMR (400 MHz, CDCl3) d 8.17 (d, J = 8.8 Hz, 1H), 7.68 (d, J = 8.8 Hz, 1H), 4.09 (s, 3H).
References:
CINCERA THERAPEUTICS PTY LTD WO2021/12018, 2021, A1 Location in patent:Page/Page column 63; 85; 90-91

63001-30-9
114 suppliers
$9.00/250mg

65202-50-8
179 suppliers
$21.21/1gm:

63001-30-9
114 suppliers
$9.00/250mg

65202-50-8
179 suppliers
$21.21/1gm:
6531-04-0
16 suppliers
$279.23/1gm:

65202-50-8
179 suppliers
$21.21/1gm:

5096-73-1
187 suppliers
$15.00/1g

124-41-4
731 suppliers
$12.00/25g

65202-50-8
179 suppliers
$21.21/1gm: