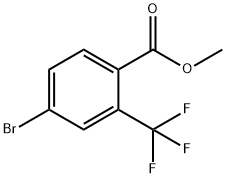
Methyl 4-bromo-2-(trifluoromethyl)benzoate synthesis
- Product Name:Methyl 4-bromo-2-(trifluoromethyl)benzoate
- CAS Number:957207-58-8
- Molecular formula:C9H6BrF3O2
- Molecular Weight:283.04

67-56-1
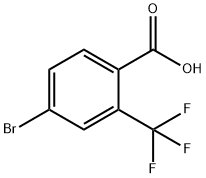
320-31-0

957207-58-8
Synthesis of methyl 4-bromo-2-(trifluoromethyl)benzoate (107): 4-bromo-2-(trifluoromethyl)benzoic acid (106; 1.6 g, 5.95 mmol) was dissolved in 20 mL of methanol. 2 mL of thionyl chloride was added slowly. After addition, the reaction mixture was stirred at 60 °C for 12 h and subsequently concentrated. To the residue was added 20 mL of ethyl acetate. The mixture was washed sequentially with saturated aqueous sodium bicarbonate and saturated brine, dried over anhydrous sodium sulfate and concentrated to give 1.6 g methyl 4-bromo-2-(trifluoromethyl)benzoate (107) as a yellow liquid. Yield 85%. LCMS: m/z 282.7 [M + H]+, tR = 1.79 min.

67-56-1
790 suppliers
$9.00/25ml

320-31-0
163 suppliers
$20.00/1g

957207-58-8
67 suppliers
$26.00/1g
Yield:957207-58-8 90%
Reaction Conditions:
with hydrogenchlorideReflux;
Steps:
A.H10
H10) 4-Bromo-2-trifluoromethyl-benzoic acid methyl ester A solution of compound H9 (25.0 g, 94 mmol) in HCl-MeOH (250 mL) was refluxed overnight. TLC showed the starting material was consumed completely. The MeOH was evaporated in vacuo. And the resulting reside was partitioned between saturated NaHCO3 and EtOAc. The organic layer was dried over anhydrous Na2SO4 and concentrated under vacuum to give product H10 (23.5 g; 90.0%).
References:
US2012/28952,2012,A1 Location in patent:Page/Page column 18-19
![[BIS(TRIFLUOROACETOXY)IODO]PENTAFLUOROBENZENE](/CAS/GIF/14353-88-9.gif)
14353-88-9
82 suppliers
$19.00/100mg
619-42-1
373 suppliers
$7.00/10g
107317-58-8
108 suppliers
$9.00/250mg

957207-58-8
67 suppliers
$26.00/1g

619-42-1
373 suppliers
$7.00/10g
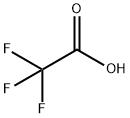
76-05-1
717 suppliers
$9.00/1g

107317-58-8
108 suppliers
$9.00/250mg

957207-58-8
67 suppliers
$26.00/1g
191165-13-6
183 suppliers
$6.00/1g

957207-58-8
67 suppliers
$26.00/1g
623-00-7
485 suppliers
$6.00/10g

957207-58-8
67 suppliers
$26.00/1g