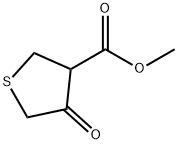
Methyl 4-oxotetrahydrothiophene-3-carboxylate synthesis
- Product Name:Methyl 4-oxotetrahydrothiophene-3-carboxylate
- CAS Number:2689-68-1
- Molecular formula:C6H8O3S
- Molecular Weight:160.19
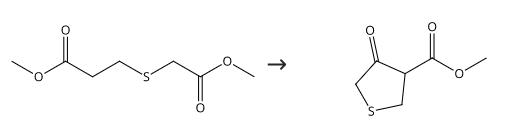
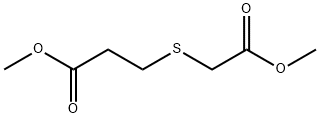
7400-45-5
70 suppliers
$35.39/1g

2689-68-1
133 suppliers
$20.00/250 mg
Yield:2689-68-1 80%
Reaction Conditions:
with pyridine in toluene at 80; for 2.5 h;
Steps:
Synthesis of 3-keto-4-carbomethoxy-2,5-tetrahydrothiophene (0)
Pyridine (0.2 mol) and toluene (500 mL) was stirred under 80°C for 10 min, then (II) (0.2 mol) was added to the solution and it was maintained at the temperature 80°C for 1h. In addition of (II) (0.2 mol) was added to the mixture in 20 min and the solution was refluxed for 1h. When cooled down to the room temperature, the samples adjusted to pH 7 with 0.5M hydrochloric acid in the ice-sodium chloride cold bath, and the toluene layer were separated. Residual solution was extracted based on ether (3*50mL),and combined with toluene layer. The mixture was evaporated to dryness in a vacuum, recrystallization from petroleum ether-anhydrous ethanol gave 38.4g of yellowish-white crystals (compound 0),Yield: 80%; mp : 36~38 C; 1H NMR(CDCl3) δ:3.75 (t,2H, J=3 Hz), 3.78 (s, 3H), 3.81 (t, 2H, J=3 H z), 10.93 (s,1H); 13C NMR (CDCl3) δ: 31.40, 38.03, 51.63, 99.21,169.55, 172.43 ppm; ESI-MS: m/z183.01 (M+Na+,100%).
References:
Tan, Chunbin;Liu, Xiaoling;Du, Hongfei [Revue Roumaine de Chimie,2019,vol. 64,# 3,p. 271 - 276]

7400-45-5
70 suppliers
$35.39/1g
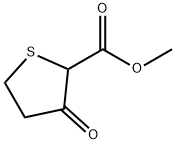
2689-69-2
95 suppliers
$50.70/250mg

2689-68-1
133 suppliers
$20.00/250 mg
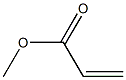
292638-85-8
5 suppliers
inquiry

2689-68-1
133 suppliers
$20.00/250 mg
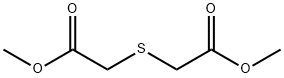
16002-29-2
108 suppliers
$6.00/1g

2689-68-1
133 suppliers
$20.00/250 mg
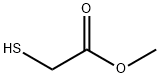
2365-48-2
369 suppliers
$15.00/25g

292638-85-8
5 suppliers
inquiry

2689-69-2
95 suppliers
$50.70/250mg

2689-68-1
133 suppliers
$20.00/250 mg