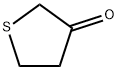
Tetrahydrothiophen-3-one synthesis
- Product Name:Tetrahydrothiophen-3-one
- CAS Number:1003-04-9
- Molecular formula:C4H6OS
- Molecular Weight:102.15
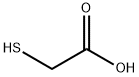
68-11-1
427 suppliers
$12.00/25g
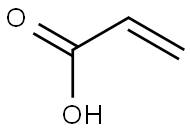
79-10-7
737 suppliers
$20.16/100 mL

1003-04-9
373 suppliers
$5.00/5g
Yield:-
Reaction Conditions:
with iron(III) chloride at 120 - 250; for 1 h;Reagent/catalyst;Temperature;
Steps:
15 Example 15
The difference from Example 1 was that the molar ratio of the raw material A to the catalyst was 1:0.05.FeCl3 (40.625 g, 0.25 mol) was dissolved in acrylic acid (360 g, 5 mol) and stirred to give a solution A. The above solution A was pumped into a Michael addition reactor (first coil, 6 m long, 6 mm in diameter) at a rate of 2.4 g/min using a first transfer pump.Using a second transfer pump, thioglycolic acid (460 g, 5 mol) was pumped into the Michael addition reactor at a rate of 3.10 g/min, and the coil was immersed in an oil bath at 120 ° C for Michael addition reaction. An addition product is obtained.The above-mentioned addition product was transferred to a Dickman condensation reactor (second coil, 6 m long, 6 mm in diameter) for Dickman condensation reaction, in which the Dickman condensation reactor was immersed in a 250 ° C oil bath. The outlet of the Dickman condensation reactor was sampled and tested by HPLC. The total reaction time was 1 h. The yield of the final product is 95% by weight.The purity was 93% by weight.
References:
Kailaiying Pharmaceutical Group (Tianjin) Co., Ltd.;Hong Hao;Lu Jiangping;Zhang Enxuan;Liu Zhiqing;Zhang Tao;Zhao Qian CN108148041, 2018, A Location in patent:Paragraph 0057; 0058; 0059; 0065; 0066; 0067; 0068; 0071
4938-00-5
10 suppliers
$144.00/1mg

1003-04-9
373 suppliers
$5.00/5g
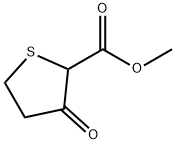
2689-69-2
95 suppliers
$50.70/250mg

1003-04-9
373 suppliers
$5.00/5g
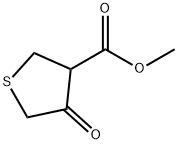
2689-68-1
133 suppliers
$20.00/250 mg

1003-04-9
373 suppliers
$5.00/5g
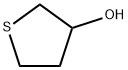
3334-05-2
59 suppliers
$121.00/250 mg

1003-04-9
373 suppliers
$5.00/5g