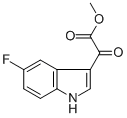
METHYL (5-FLUORO-1H-INDOL-3-YL)(OXO)ACETATE synthesis
- Product Name:METHYL (5-FLUORO-1H-INDOL-3-YL)(OXO)ACETATE
- CAS Number:408356-39-8
- Molecular formula:C11H8FNO3
- Molecular Weight:221.18
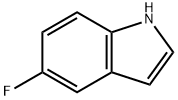
399-52-0
381 suppliers
$10.00/1g

79-37-8
490 suppliers
$17.67/10gm:

124-41-4
730 suppliers
$12.00/25g

408356-39-8
24 suppliers
$276.14/250mg
Yield:408356-39-8 87%
Reaction Conditions:
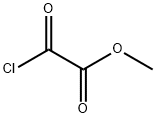
Stage #1: 5-fluoro-1H-indole;oxalyl dichloride in diethyl ether at 0; for 0.5 h;
Stage #2: sodium methylate in methanol;diethyl ether at -78 - 20;
Steps:
Methyl 2-(5-fluoro-1H-indol-3-yl) -2--oxoacetate (5a)
To a solution of 5-fluoro-lH-indole (2.00 g, 14.8 mmol, 1.00 equiv) in Et20 (20.0 mL, 0.740 M) was added oxalyl chloride (1.80 mL, 21.1 mmcl, 1.43 equiv) dropwise at 0 °C. The yellow slurry was stirred at0 °C for 30 mm and then cooled to -78 00. A solution of NaOMe in MeOH (25%, 8.00 mL) was added to this slurry at the same temperature. The reaction mixture was then allowed to warm up to room temperature, and was quenched by addition of water (10.0 mL) . The precipitate was collected by filtration, washed with water and dried to afford thetitle compound as a yellow solid (2.85 g, 12.9 mmol, 87% yield). NMR Spectroscopy: ‘H NMR (700 MHz, (CD,),SO, 25 °C, ): 12.54 (s, 1H), 8.51 (s, 1H), 7.82 (s, 1H), 7.56 (s, lH), 7.12-7.20 (m, lH), 3.89 (s,3H) . “C NMR (175 MHz, (CD,)2S0, 25 °C, ) : 178.4, 163.6, 159.2 (d, J232.8 Hz), 139.7, 133.3, 126.3 (d, J = 10.5 Hz), 114.2 (d, J = 10.5Hz), 112.5 (d, J= 5.3 Hz), 112.0 (d, J= 26.3 Hz), 106.2 (d, J= 24.5Hz), 52.6. The ‘H NMR data were in good agreement with values reportedin the literature (Ye, Q. et al. 2015)
References:
WO2018/132636,2018,A1 Location in patent:Page/Page column 40
5781-53-3
246 suppliers
$8.00/10g

399-52-0
381 suppliers
$10.00/1g

408356-39-8
24 suppliers
$276.14/250mg

399-52-0
381 suppliers
$10.00/1g

79-37-8
490 suppliers
$17.67/10gm:

408356-39-8
24 suppliers
$276.14/250mg

124-41-4
730 suppliers
$12.00/25g
3828-09-9
1 suppliers
inquiry

408356-39-8
24 suppliers
$276.14/250mg

67-56-1
790 suppliers
$9.00/25ml

3828-09-9
1 suppliers
inquiry

408356-39-8
24 suppliers
$276.14/250mg