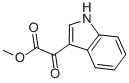
Methyl indolyl-3-glyoxylate synthesis
- Product Name:Methyl indolyl-3-glyoxylate
- CAS Number:18372-22-0
- Molecular formula:C11H9NO3
- Molecular Weight:203.19
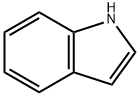
120-72-9

79-37-8

124-41-4

18372-22-0
To a three-necked flask equipped with a magnetic stirrer and two addition funnels were added indole (10.1 g, 0.086 mol) and 100 mL of diethyl ether. Oxalyl chloride (7.3 mL, 0.086 mol) was added slowly and dropwise over 0.5 h at 0 °C under nitrogen protection. A yellow precipitate appeared in the reaction mixture and stirring was continued for 0.5 hr. Subsequently, the reaction mixture was cooled to -70 °C via a dry ice bath and sodium methanolate (25% methanol solution, 37.3 g, 0.173 mol) was added dropwise over 1 hour. After the dropwise addition, the reaction mixture was slowly warmed to 0 °C and 50 mL of water was added. The precipitate was collected by filtration, washed with water several times, and dried under vacuum at 60 ℃ to obtain methyl indole-3-glyoxylate as a yellow powder with 90% yield, which could be used in the next reaction without further purification. In another three-necked flask equipped with a magnetic stirrer and a dosing funnel, 3-indoleacetamide (8.0 g, 0.046 mol), indole-3-glyoxalic acid methyl ester (10.0 g, 0.049 mol) and 80 mL of tetrahydrofuran were added. A solution of potassium tert-butanolate (15.2 g, 0.135 mol) in 130 mL of tetrahydrofuran was slowly added dropwise over a period of 1.5 h at 0 °C under nitrogen protection. After the dropwise addition, the reaction mixture was slowly warmed to room temperature and stirring was continued for 3 hours. Subsequently, a 35% aqueous hydrochloric acid solution (64 mL) was added slowly and dropwise over 1 hour. Upon completion of the reaction, 200 mL of ethyl acetate and 100 mL of water were added and stirred until completely dissolved. The organic phase was separated, washed sequentially with water to neutral, brine once, and dried with anhydrous sodium sulfate. The desiccant was removed by filtration and the filtrate was concentrated. Add 1:1 (v/v) mixed solvent of ethyl acetate and n-hexane dropwise to the concentrated solution, and crystallize at 50~60°C to obtain 3,4-bisindolylmaleimide pure as red crystals.

120-72-9
658 suppliers
$6.00/25g

67-56-1
790 suppliers
$7.29/5ml-f

79-37-8
490 suppliers
$17.67/10gm:

18372-22-0
151 suppliers
$8.00/250mg
Yield: 90%
Reaction Conditions:
Stage #1:indole;oxalyl dichloride in diethyl ether at 0 - 20; for 6 h;
Stage #2:methanol in diethyl ether at 0; for 0.5 h;
Steps:
Preparation of compound methyl 2-(1H-indol-3-yl)-2-oxoacetate (1)
In a reaction flask,7 g ( 60 mmol ) indole and 300 mL of dry diethyl ether were add, and 16.1 mL of oxalyl chloride was added dropwise to the reaction solution at 0 °C. The reaction was continued for 6 h at room temperature to form a yellow suspension. It was further placed at 0° C, 12 mL of methanol was added dropwise to the reaction, and the mixture was stirred for 30 min. The resulting mixture was filtered and washed with ice ether to finally obtain a yellow solid 1 ( 10.93 g , 90% yield).
References:
Guo, Zhenbo;Xu, Yiming;Peng, Yujie;Haroon ur Rashid;Quan, Wei;Xie, Peng;Wu, Lichuan;Jiang, Jun;Wang, Lisheng;Liu, Xu [Bioorganic and Medicinal Chemistry Letters,2019,vol. 29,# 9,p. 1133 - 1137] Location in patent:supporting information

120-72-9
658 suppliers
$6.00/25g

79-37-8
490 suppliers
$17.67/10gm:

124-41-4
731 suppliers
$12.00/25g

18372-22-0
151 suppliers
$8.00/250mg

67-56-1
790 suppliers
$7.29/5ml-f
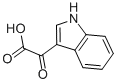
1477-49-2
135 suppliers
$10.00/100mg

18372-22-0
151 suppliers
$8.00/250mg
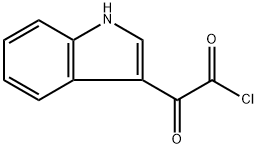
22980-09-2
77 suppliers
$39.00/1g

124-41-4
731 suppliers
$12.00/25g

18372-22-0
151 suppliers
$8.00/250mg

120-72-9
658 suppliers
$6.00/25g

18372-22-0
151 suppliers
$8.00/250mg