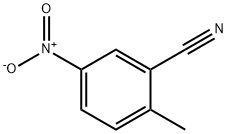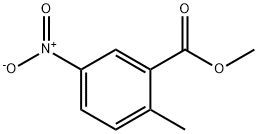
Methyl 5-nitro-2-methylbenzoate synthesis
- Product Name:Methyl 5-nitro-2-methylbenzoate
- CAS Number:77324-87-9
- Molecular formula:C9H9NO4
- Molecular Weight:195.17
1975-52-6

74-88-4

77324-87-9
4.2.1 Synthesis of methyl 2-methyl-5-nitrobenzoate (2): 2-methyl-5-nitrobenzoic acid (15 g, 82.8 mmol) and potassium carbonate (K2CO3, 17.2 g, 124.2 mmol) were suspended in N,N-dimethylformamide (DMF, 75 mL) in a dry round-bottom flask. To this suspension, iodomethane (6.7 mL) was slowly added dropwise at room temperature. The reaction mixture was stirred at room temperature overnight (~12 hours). After completion of the reaction, the mixture was poured into water (500 mL) and the aqueous phase was extracted three times with ethyl acetate (EA). The organic phases were combined, dried with anhydrous sodium sulfate (Na2SO4), filtered and concentrated under reduced pressure to give methyl 2-methyl-5-nitrobenzoate (15.3 g, 94% yield) as a yellow solid. The product was confirmed by 1H NMR (400 MHz, CDCl3): δ 8.52 (s, 1H), 8.05 (s, 1H), 7.30 (s, 1H), 3.83 (s, 3H), 2.56 (s, 3H). ESI-MS analysis showed m/z 196 [M + H]+.

67-56-1
790 suppliers
$7.29/5ml-f
201230-82-2
1 suppliers
inquiry
7745-92-8
155 suppliers
$9.00/1g

77324-87-9
213 suppliers
$6.00/1g
Yield: 37%
Reaction Conditions:
with triethylamine;diphenylphosphinopropane;palladium diacetate in methanol at 110; under 3000.3 Torr; for 18 h;
Steps:
104.a EXAMPLE 104; (E)-3-Ethyl-7-[3-(3-hydroxymethyl-4-methylphenoxymethyl)phenyl]non-6-en-3-ol; [01155] a) Methyl 2-Methyl-5-nitrobenzoate
23.2 g (88 mmol) of 2-iodo-4-nitrotoluene are dissolved in 400 ml of anhydrous methanol and then placed in a steel reactor. 25 ml (177 mmol) of triethylamine, 1.97 g (8.8 mmol) of palladium diacetate and 7.3 g of diphenyphosphinopropane are added and the reaction medium is subjected to a carbon monoxide pressure of 4 bar and heated at 110° C. for 18 hours. The medium is then brought to ambient pressure and temperature, dissolved in dichloromethane and filtered on celite. After evaporation, the residue is purified by chromatography on a silica column. An orange-coloured powder is obtained (m=6.3 g, Y=37%).
References:
Galderma Research & Development S.N.C. US6689922, 2004, B1 Location in patent:Page column 86

1975-52-6
285 suppliers
$12.00/1 g

74-88-4
357 suppliers
$15.00/10g

77324-87-9
213 suppliers
$6.00/1g

67-56-1
790 suppliers
$7.29/5ml-f

1975-52-6
285 suppliers
$12.00/1 g

77324-87-9
213 suppliers
$6.00/1g

1975-52-6
285 suppliers
$12.00/1 g

77324-87-9
213 suppliers
$6.00/1g