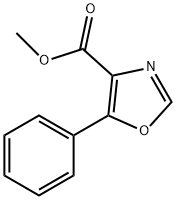
Methyl 5-phenyloxazole-4-carboxylate ,97% synthesis
- Product Name:Methyl 5-phenyloxazole-4-carboxylate ,97%
- CAS Number:38061-18-6
- Molecular formula:C11H9NO3
- Molecular Weight:203.19
Yield:38061-18-6 67%
Reaction Conditions:
with triethylamine in tetrahydrofuran at 20; for 48 h;
Steps:
23
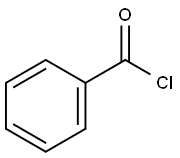
Reference Example 23: Production of 6g; [Show Image] To THF (tetrahydrofuran) (50 mL), methylisocyanoate (2.97 g, 30 mmol), benzoyl chloride (2.97 g, 30 mmol) and TEA (triethyl amine) (12.6 mL, 90 mmol) were added, and stirred at room temperature for 48 hours. Then, the solvent was distilled off under reduced pressure, and ethyl acetate (100 ml) was added in the residue, and washed with water, 1 mol/L HCl (50 ml), saturated NaHCO3 (50 ml) and saturated salt water (50 ml) in that order. After drying the solution over anhydrous sodium sulfate, precipitates were filtered off, and the solvent was distilled off under reduced pressure. The residue was purified with silica gel column chromatography (100 g, ethyl acetate : n-hexane = 1:5) to obtain an oxazole compound (4.07 g, 20 mmol, 67%) as colorless solid. 1H-NMR (400 MHz, CDCl3) δ 3.96 (s, 3H), 7.45-7.53 (3H, m, Ar-H), 7.92 (s, 1H, oxazole-H), 8.00-8.12 (2H, m, Ar-H); FT-IR νmax (KBr): 3108, 1717, 1582, 1561, 1516, 1495, 1433, 1354, 1325, 1312, 1221, 1195, 1109, 1087, 1068, 1010, 936, 767, 688.
References:
EP1650185,2006,A1 Location in patent:Page/Page column 20-21
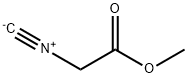
39687-95-1
149 suppliers
$33.81/1g

100-52-7
972 suppliers
$20.37/250g

38061-18-6
13 suppliers
inquiry

39687-95-1
149 suppliers
$33.81/1g
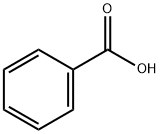
65-85-0
1439 suppliers
$10.00/25g

38061-18-6
13 suppliers
inquiry