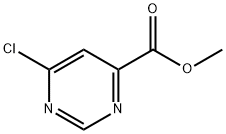
methyl 6-chloropyrimidine-4-carboxylate synthesis
- Product Name:methyl 6-chloropyrimidine-4-carboxylate
- CAS Number:6627-22-1
- Molecular formula:C6H5ClN2O2
- Molecular Weight:172.57
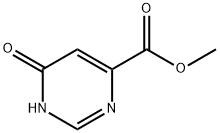
7399-93-1

6627-22-1
General procedure for the synthesis of methyl 6-chloropyrimidine-4-carboxylate from methyl 6-oxo-3,6-dihydropyrimidine-4-carboxylate: [0778] To a 2000 mL four-necked round-bottomed flask was added methyl 6-hydroxypyrimidine-4-carboxylate (115 g, 746.16 mmol, 1.00 equiv.), acetonitrile (1,200 mL), and phosphorous triclopyr (340 g, 2.22 mol, 3.00 equiv). The reaction mixture was stirred at 80 °C overnight. Upon completion of the reaction, the mixture was cooled to room temperature and concentrated under vacuum. Subsequently, the reaction mixture was diluted with 1000 mL of ethyl acetate and quenched with 1000 mL of ice water. The aqueous phase was extracted with 3 x 500 mL of ethyl acetate. The organic layers were combined, washed sequentially with 1000 mL of water and 1000 mL of saturated saline, dried over anhydrous sodium sulfate, and concentrated under vacuum. The residue was purified by silica gel column chromatography using ethyl acetate/petroleum ether (1:4) as eluent to give 76 g (59% yield) of methyl 6-chloropyrimidine-4-carboxylate as a white solid.LCMS [M + H]+ 173.

7399-93-1
90 suppliers
$31.00/250mg

6627-22-1
126 suppliers
$28.00/250mg
Yield: 76 g
Reaction Conditions:
with trichlorophosphate in acetonitrile at 80;
Steps:
7.2 Step 2: Preparation of methyl 6-chloropyrimidine-4-carboxylate
[0778] Into a 2000-mL 4-necked round-bottom flask was placed methyl 6-hydroxypyrimidine-4- carboxylate (115 g, 746.16 mmol, 1.00 equiv), CH3CN (1200 mL), and POC13 (340 g, 2.22 mol, 3.00 equiv). The resulting solution was stirred at 80°C overnight, cooled to room temperature, concentrated under vacuum, diluted with 1000 mL of EA, and quenched with 1000 mL of water/ice. The resulting solution was extracted with 3x500 mL of ethyl acetate. The combined organic layers were washed with lx 1000 mL of water and lx 1000 mL of brine, dried over anhydrous sodium sulfate, and concentrated under vacuum. The residue was applied onto a silica gel colunm eluted with ethyl acetate/petroleum ether (1:4) to afford 76 g (59%) of methyl 6-chloropyrimidine-4-carboxylate as a white solid. LCMS [M+H] 173.
References:
F. HOFFMANN-LA ROCHE AG;GENENTECH, INC.;ESTRADA, Anthony;VOLGRAF, Matthew;CHEN, Huifen;KOLESNIKOV, Aleksandr;VILLEMURE, Elisia;VERMA, Vishal;WANG, Lan;SHORE, Daniel;DO, Steven;YUEN, Po-wai;HU, Baihua;WU, Guosheng;LIN, Xingyu;LU, Aijun WO2016/128529, 2016, A1 Location in patent:Paragraph 0774; 0777; 0778
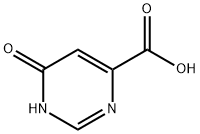
6299-87-2
202 suppliers
$6.00/100mg

6627-22-1
126 suppliers
$28.00/250mg