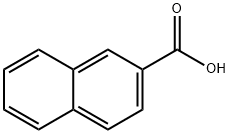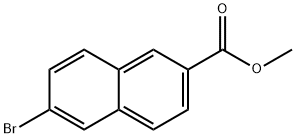
Methyl 6-bromo-2-naphthoate synthesis
- Product Name:Methyl 6-bromo-2-naphthoate
- CAS Number:33626-98-1
- Molecular formula:C12H9BrO2
- Molecular Weight:265.1

67-56-1
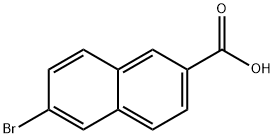
5773-80-8

33626-98-1
To a 60 mL dry egg-shaped flask was added 6-bromo-2-naphthalenecarboxylic acid (2.4996 g, 10.0 mmol) followed by 20 mL of anhydrous methanol to dissolve it. Slowly 1 mL of concentrated sulfuric acid was added dropwise to the reaction system and then the mixture was refluxed overnight. The progress of the reaction was monitored by TLC until complete conversion of the feedstock. After completion of the reaction, the heating was stopped and the system was allowed to cool to room temperature. The reaction was quenched with saturated aqueous sodium carbonate and the reaction system was adjusted to neutral. The reaction mixture was extracted with ethyl acetate and the organic phase was washed with saturated aqueous sodium carbonate, water and saturated aqueous sodium chloride, respectively. The organic layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure to give methyl 6-bromo-2-naphthalenecarboxylate (S7, 2.63 g, 100% yield) as a white solid.

67-56-1
790 suppliers
$9.00/25ml

5773-80-8
306 suppliers
$10.00/1g

33626-98-1
322 suppliers
$16.80/1g
Yield:33626-98-1 100%
Reaction Conditions:
with sulfuric acidReflux;
Steps:
2 Synthesis of S7
6-Bromo-2-naphthalenecarboxylic acid (2.4996 g, 10.0 mmol) was added50mL dried egg-shaped flask, add 20mL of anhydrous methanol to dissolve,Then 1 mL of concentrated sulfuric acid was slowly added dropwise and the system was refluxed overnight.TLC tracking until the conversion of raw materials is completed, stop heating,After cooling to room temperature, saturated aqueous sodium carbonate solution was added to quench the reaction.The reaction system was adjusted to neutrality, extracted with ethyl acetate,The organic phase is washed three times with anhydrous sodium sulfate.Concentration under reduced pressure afforded S7 (2.63 g, 100% yield) as a white solid.
References:
CN107286150,2017,A Location in patent:Paragraph 0153; 0154; 0155; 0156; 0157
51934-38-4
44 suppliers
$456.00/250mg

74-88-4
357 suppliers
$15.00/10g

33626-98-1
322 suppliers
$16.80/1g
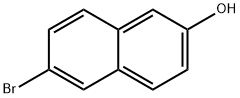
15231-91-1
405 suppliers
$7.00/5g

33626-98-1
322 suppliers
$16.80/1g

67-56-1
790 suppliers
$9.00/25ml
201230-82-2
1 suppliers
inquiry
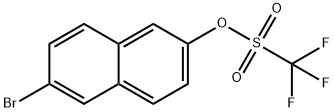
151600-02-1
58 suppliers
$23.00/250mg

33626-98-1
322 suppliers
$16.80/1g