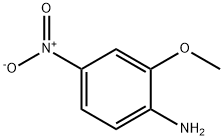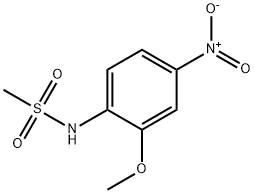
N-(2-METHOXY-4-NITRO-PHENYL)-METHANESULFONAMIDE synthesis
- Product Name:N-(2-METHOXY-4-NITRO-PHENYL)-METHANESULFONAMIDE
- CAS Number:57164-98-4
- Molecular formula:C8H10N2O5S
- Molecular Weight:246.24
Yield:57164-98-4 98%
Reaction Conditions:
with pyridine at 0 - 20; for 3 h;Product distribution / selectivity;
Steps:
4.A; 6.A; 8.A
A) 2-Methoxy-4-nitro-methanesulfonylaminobenzene.; To a solution of 2-methoxy-4-nitroaniline (5 g) in pyridine (25 ml) at 0°C was added dropwise methanesulfonyl chloride (3.5 ml) then pyridine (0.5 ml). The mixture was left at 0°C for 1 hour, then brought to room temperature for 2 h. The mixture was poured onto ice (100 g) and dilute hydrochloric acid (3M, 100 ml), the solid formed was filtered, then washed with water to give 2-Methoxy-4-nitromethanesulfonylaminobenzene (7.32 g, . 98%) as an off-white crystalline solid. 1H NMR (CDC13) 7.92 (1 H, dd, J 2, 8Hz), 7.78 (IH, d, J 2Hz), 7.64 (1 H, d, J 8Hz) and 7.23 (1H, bs, NH). A) 2-Methoxy-4-nitro-methanesulfonylaminobenzene.; To a solution of2-methoxy-4-nitroaniline (5 g) in pyridine (25 ml) at 0°C was added dropwise methanesulfonyl chloride (3.5 ml).The mixture was left at 0°C for 1 hour, then brought to RT for 2 h. The mixture was poured onto ice (100 g) and dilute hydrochloric acid (3M, 100 ml), the solid formed was filtered then washed with water and dried to give 2-methoxy-4-nitro-methanesulfonylaminobenzene (7.32 g, 98%)as an off-white crystalline solid. 1H NMR (CDC13) 7.93 (1H, dd, J 2,8Hz), 7.78 (1H, d, J 2Hz), 7.65 (1H, d, J 8Hz), 7.23 (1H, bs), 4.00 (3H, s) and 3.09 (3H, s). A) 2-Methoxy-4-nitro-methanesulfonylaminobenzene.; To a solution of 2-methoxy-4-nitroaniline (5 g) in pyridine (25 ml) at 0°C was added dropwise methanesulfonyl chloride (3.5 ml).The mixture was left at 0°C for 1 hour, then brought to RT for 2 h. The mixture was poured onto ice (100 g) and dilute hydrochloric acid (3M, 100 ml), the solid formed was filtered then washed with water and dried to give 2-methoxy-4-nitro-methanesulfonylaminobenzene (7.32 g, 98%)as an off-white crystalline solid. 'H NMR (CDC13) 7.93 (1H, dd, J 2,8Hz), 7.78 (1H, d, J 2Hz), 7.65 (1H, d, J 8Hz), 7.23 (1H, bs), 4.00 (3H, s) and 3.09 (3H, s).
References:
WO2005/113489,2005,A1 Location in patent:Page/Page column 73; 78; 83-84
7022-25-5
5 suppliers
inquiry

57164-98-4
13 suppliers
$106.00/25MG
90-04-0
393 suppliers
$5.00/5G

57164-98-4
13 suppliers
$106.00/25MG