
N-(3-AMINOPHENYL)-2-METHYLBENZAMIDE synthesis
- Product Name:N-(3-AMINOPHENYL)-2-METHYLBENZAMIDE
- CAS Number:293737-97-0
- Molecular formula:C14H14N2O
- Molecular Weight:226.27
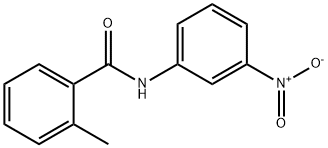
22978-51-4
2 suppliers
inquiry

293737-97-0
24 suppliers
$45.00/10mg
Yield:293737-97-0 80%
Reaction Conditions:
with iron;calcium chloride in ethanol;water at 60;
Steps:
3.4.2. General procedures for preparation of compounds (2a-e):
General procedure: Dissolve 1 eq. of nitro compound in 10 mL of Ethanol, 0.5 mL ofwater and then iron powder (3 eq.) and Calcium chloride (CaCl2) (1 eq.)were added to the reaction mixture. The resulting reaction mixture wasallowed to reflux at 60 °C for 4-5 h. Progress of the reaction wasmonitored by TLC. Spots on TLC plates were visualized under UV lampor by staining it with 0.2% ninhydrin in ethanol solution and charringafter elution. After completion, the reaction mixture was filtered toremove the iron residues; the recovery of the reaction product is performedby evaporating the solvent under reduced pressure. The resultingresidue is then taken up in ethyl acetate. The organic extractswere washed with H2O (3×10 mL), brine (2×10 mL), and dried overNa2SO4, the organic phase was evaporated. Purification of the compoundhas been performed by Silica column chromatography usingHexane: Ethyl acetate (80:20) as eluting solvent.22
References:
Qureshi, Shahnawaz I.;Chaudhari, Hemchandra K. [Bioorganic and Medicinal Chemistry,2019,vol. 27,# 12,p. 2676 - 2688] Location in patent:supporting information
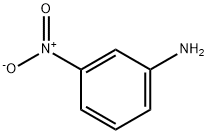
99-09-2
36 suppliers
$14.00/1g

293737-97-0
24 suppliers
$45.00/10mg
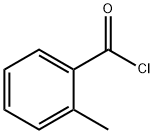
933-88-0
356 suppliers
$10.00/1g

293737-97-0
24 suppliers
$45.00/10mg
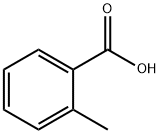
118-90-1
533 suppliers
$13.00/1g

293737-97-0
24 suppliers
$45.00/10mg
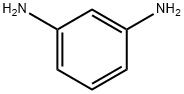
108-45-2
484 suppliers
$13.00/25g

293737-97-0
24 suppliers
$45.00/10mg