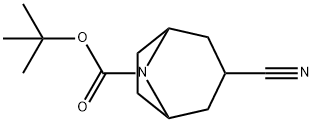
N-Boc-3-Cyano-8-azabicyclo[3.2.1]octane synthesis
- Product Name:N-Boc-3-Cyano-8-azabicyclo[3.2.1]octane
- CAS Number:856900-26-0
- Molecular formula:C13H20N2O2
- Molecular Weight:236.31
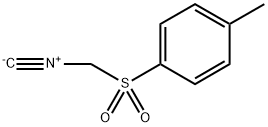
36635-61-7
522 suppliers
$5.00/5g
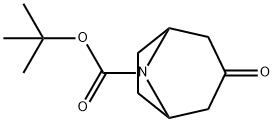
185099-67-6
246 suppliers
$5.00/250mg
![N-Boc-3-Cyano-8-azabicyclo[3.2.1]octane](/CAS/GIF/856900-26-0.gif)
856900-26-0
38 suppliers
inquiry
Yield:856900-26-0 57.96%
Reaction Conditions:
with potassium tert-butylate in 1,2-dimethoxyethane;ethanol at 0 - 60; for 16 h;Inert atmosphere;
Steps:
35A Example 35A tert-butyl-3-cyano-8-azabicyclo[3.2.1]octane-8-carboxylate
Example 35A
tert-butyl-3-cyano-8-azabicyclo[3.2.1]octane-8-carboxylate
To a mixture of tert-butyl 3-oxo-8-azabicyclo[3.2.1]octane-8-carboxylate (10 g, 44.389 mmol) in DME (300 mL) and EtOH (6.7 mL) was added portionwise t-BuOK (20 g, 177.557 mmol) and TOSMIC (17.33 g, 88.778 mmol) at 0° C. under N2 atmosphere.
After being stirred at 60° C. for 16 h, the resultant mixture was quenched with water (100 mL).
The aqueous layer was extracted with EtOAc (100 mL*2).
The combined organic layers were washed with water, brine, dried over Na2SO4, filtered and evaporated.
The residue was purified by column chromatograph to provide the title compound (6.08 g, yield: 57.96%) as a white solid. LCMS (ESI) m/z: 327 (M+1).
References:
US2017/29430,2017,A1 Location in patent:Paragraph 0392
![Benzene, 1-[(isocyanatomethyl)sulfonyl]-4-methyl-](/CAS/20180601/GIF/10564-55-3.gif)
10564-55-3
0 suppliers
inquiry

185099-67-6
246 suppliers
$5.00/250mg
![N-Boc-3-Cyano-8-azabicyclo[3.2.1]octane](/CAS/GIF/856900-26-0.gif)
856900-26-0
38 suppliers
inquiry
![tert-Butyl 3-hydroxy-8-azabicyclo[3.2.1]octane-8-carboxylate](/CAS/20180629/GIF/478837-18-2.gif)
478837-18-2
42 suppliers
$21.00/250mg

36635-61-7
522 suppliers
$5.00/5g
![N-Boc-3-Cyano-8-azabicyclo[3.2.1]octane](/CAS/GIF/856900-26-0.gif)
856900-26-0
38 suppliers
inquiry

185099-67-6
246 suppliers
$5.00/250mg
![N-Boc-3-Cyano-8-azabicyclo[3.2.1]octane](/CAS/GIF/856900-26-0.gif)
856900-26-0
38 suppliers
inquiry
25602-68-0
208 suppliers
$11.00/5g
![N-Boc-3-Cyano-8-azabicyclo[3.2.1]octane](/CAS/GIF/856900-26-0.gif)
856900-26-0
38 suppliers
inquiry