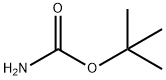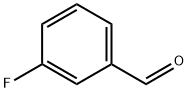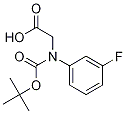
N-Boc-DL-3-FluoroPhenylglycine synthesis
- Product Name:N-Boc-DL-3-FluoroPhenylglycine
- CAS Number:142121-94-6
- Molecular formula:C13H16FNO4
- Molecular Weight:269.27

24424-99-5
871 suppliers
$13.50/25G
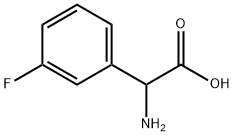
7292-74-2
72 suppliers
$19.00/100mg

142121-94-6
18 suppliers
inquiry
Yield:142121-94-6 69%
Reaction Conditions:
Stage #1: di-tert-butyl dicarbonate;DL-2-(3-fluorophenyl)glycinewith sodium hydroxide in tetrahydrofuran;water at 20; for 15 h;
Stage #2: with hydrogenchloride in water; pH=1;Cooling;
Steps:
19
To a suspension of 2-amino-2-(3-fluorophenyl)acetic acid (1.00 g, 5.91 mmol) in THF (30 ml) and water (30 ml), 2N sodium hydroxide (29.6 ml, 59.1 mmol) and di-tert-butyl dicarbonate (2.58 g, 1 1.8 mmol) were added and the reaction was stirred at RT for 15h. THF was evaporated, the aqueous phase was cooled and acidified with 37% HCl until pH 1. The desired compound was extracted with EtOAc and the organic phase was washed with brine, dried over Na2SO4 and evaporated to obtain 2-(tert-butoxycarbonylamino)-2-(3- fluorophenyl)acetic acid (1.10 g; 69% yield).
References:
WO2012/69275,2012,A1 Location in patent:Page/Page column 64-65
501-00-8
268 suppliers
$21.00/5g

142121-94-6
18 suppliers
inquiry

124-38-9
134 suppliers
$214.00/14L
![Carbamic acid, N-[(3-fluorophenyl)(phenylsulfonyl)methyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/1237534-69-8.gif)
1237534-69-8
0 suppliers
inquiry

142121-94-6
18 suppliers
inquiry

153903-29-8
4 suppliers
inquiry