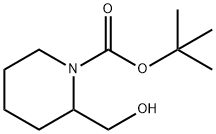
N-Boc-piperidine-2-methanol synthesis
- Product Name:N-Boc-piperidine-2-methanol
- CAS Number:157634-00-9
- Molecular formula:C11H21NO3
- Molecular Weight:215.29

24424-99-5
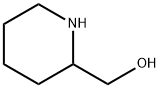
3433-37-2

157634-00-9
The general procedure for the synthesis of 1-Boc-2-piperidinemethanol from di-tert-butyl dicarbonate and 2-piperidinemethanol is as follows: 1. 2-Piperidinemethanol (3.0 g, 25.60 mmol) was dissolved in dichloromethane (DCM, 100 mL) under stirring. 2. triethylamine (14.5 mL, 91.08 mmol) was added to the above solution in batches, followed by di-tert-butyl dicarbonate (7.2 g, 31.0 mmol). 3. The reaction mixture was stirred at room temperature for 16 hours. 4. Upon completion of the reaction, water (70 mL) was added for stratification to separate the organic layer. 5. The organic layer was washed with water (2 x 50 mL) and then dried with anhydrous sodium sulfate (Na2SO4). 6. The dried organic layer was filtered and concentrated under vacuum to give 1-Boc-2-piperidinemethanol (6.0 g, quantitatively) as a light brown oil, which can be used without further purification. The nuclear magnetic resonance hydrogen spectrum (1H NMR, CDCl3) data were as follows: δ 4.42-4.22 (1H, m), 4.05-3.91 (1H, m), 3.89-3.77 (1H, m), 3.71-3.53 (1H, m), 2.97-2.81 (1H, m), 2.32-2.08 (1H, m), 1.90 (1H, s ), 1.77-1.57 (3H, m), 1.54 (1H, s), 1.52-1.38 (10H, m).

24424-99-5
871 suppliers
$13.50/25G

3433-37-2
241 suppliers
$7.00/5g

157634-00-9
201 suppliers
$5.00/1g
Yield:157634-00-9 100%
Reaction Conditions:
with triethylamine in dichloromethane at 20; for 16 h;
Steps:
INTERMEDIATE 28 (METHOD Z); ferf-Butyl 2-(hydroxymethyl*)piperidine- 1 -carboxylate; To a stirred solution of 2-piperidinemethanol (3.0 g, 25.60 mmol) in DCM (100 mL) was added triethylamine (14.5 mL, 91.08 mmol) and then di-tert-butyl dicarbonate (7.2 g, 31.0 mmol) portionwise. The reaction mixture was stirred at r.t. for 16 h. Water (70 mL) was added and the layers were separated. The organic layer was washed with water (2 x 50 mL), dried (Na2SO4), filtered and concentrated in vacuo to give the title compound (6.0 g, quantitative) as a pale brown oil that was used without further purification. δH (CDCl3) 4.42-4.22 (IH, m), 4.05-3.91 (IH, m), 3.89-3.77 (IH, m), 3.71- 3.53 (IH, m), 2.97-2.81 (IH, m), 2.32-2.08 (IH, m), 1.90 (IH, s), 1.77-1.57 (3H, m), 1.54 (IH, s), 1.52-1.38 (1OH, m).
References:
WO2007/141504,2007,A1 Location in patent:Page/Page column 57
98303-20-9
231 suppliers
$11.19/5G

157634-00-9
201 suppliers
$5.00/1g
541-16-2
254 suppliers
$14.00/5g

3433-37-2
241 suppliers
$7.00/5g

157634-00-9
201 suppliers
$5.00/1g
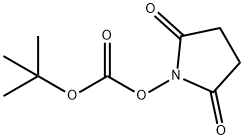
13139-12-3
175 suppliers
$5.00/1g

3433-37-2
241 suppliers
$7.00/5g

157634-00-9
201 suppliers
$5.00/1g
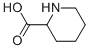
4043-87-2
246 suppliers
inquiry

157634-00-9
201 suppliers
$5.00/1g