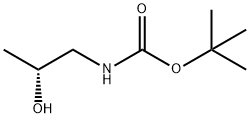
N-BOC-(R)-1-AMINO-2-PROPANOL synthesis
- Product Name:N-BOC-(R)-1-AMINO-2-PROPANOL
- CAS Number:119768-44-4
- Molecular formula:C8H17NO3
- Molecular Weight:175.23

24424-99-5
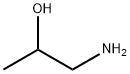
78-96-6
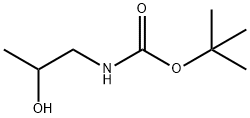
95656-86-3
Step 1: 1-Amino-2-propanol (compound 42.1; 3.53 g, 47.0 mmol) and triethylamine (25 mL) were dissolved in methanol (35 mL) under nitrogen protection. Subsequently, a solution of di-tert-butyl dicarbonate (10.3 g, 47.0 mmol) in methanol (15 mL) was added slowly and dropwise. The reaction mixture was stirred overnight at room temperature. Upon completion of the reaction, the mixture was concentrated and the residue was dried under high vacuum to give 8.23 g (quantitative yield) of clear oily product. This oily product was dissolved in tetrahydrofuran (100 mL) and triethylamine (13.1 mL, 94.0 mmol) was added. Methanesulfonyl chloride (3.82 mL, 49.3 mmol) was added dropwise to this solution at 0 °C. After 1 h of reaction, the mixture was diluted with ethyl acetate and washed sequentially with 1 M aqueous hydrochloric acid, aqueous sodium bicarbonate and brine. The organic phase was dried with anhydrous sodium sulfate and concentrated to give 10.5 g (88% yield) of a clear oily product (compound 42.2), which solidified on standing. Electrospray mass spectrometry (ES(+)MS) showed m/e = 254 ([M+H]+).
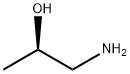
2799-16-8
273 suppliers
$5.00/250mg

24424-99-5
868 suppliers
$13.50/25G

119768-44-4
102 suppliers
$13.00/1g
Yield:119768-44-4 100%
Reaction Conditions:
in dichloromethane at 0 - 20; for 2 h;Inert atmosphere;
Steps:
Intermediate 3 (1-3) (2i?-Hydroxy-propyl)-carbamic acid tert- vXy\ ester (1-3)
Intermediate 3 (1-3) (2i?-Hydroxy-propyl)-carbamic acid tert- vXy ester (1-3) Di-fert-butyl dicarbonate (58.1 g, 266.3 mmol) in DCM (50 mL) was added to a stirred solution of (i?)-(-)-l-amino-2-propanol in DCM (50 mL) at 0 °C under nitrogen. The mixture was stirred at rt for 2 h. The mixture was diluted with cooled water and extracted with DCM. The organic layer was separated, dried (Na2S04), filtered and the solvents evaporated in vacuo to yield intermediate 1-3 as a colorless oil (47 g, quant.). The product was used in the next step without further purification.
References:
JANSSEN PHARMACEUTICA NV;VAN GOOL, Michiel, Luc, Maria;ALONSO-DE DIEGO, Sergio-Alvar;CID-NÚÑEZ, José, Maria;DELGADO-GONZÁLEZ, Óscar;DECORTE, Annelies, Marie, Antonius;MACDONALD, Gregor, James;MEGENS, Antonius, Adrianus, Hendrikus, Petrus;TRABANCO-SUÁREZ, Andrés, Avelino;GARCÍA-MOLINA, Aránzazu;ANDRÉS-GIL, José, Ignacio WO2014/195311, 2014, A1 Location in patent:Page/Page column 78

2799-16-8
273 suppliers
$5.00/250mg
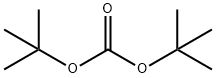
34619-03-9
34 suppliers
inquiry

119768-44-4
102 suppliers
$13.00/1g
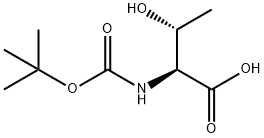
2592-18-9
323 suppliers
$5.00/1g

119768-44-4
102 suppliers
$13.00/1g