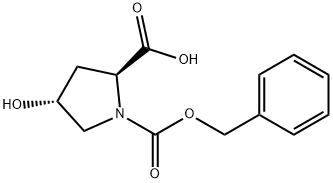
N-Cbz-Hydroxy-L-proline synthesis
- Product Name:N-Cbz-Hydroxy-L-proline
- CAS Number:13504-85-3
- Molecular formula:C13H15NO5
- Molecular Weight:265.26

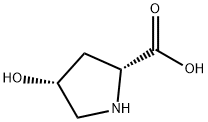
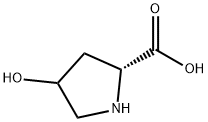
501-53-1
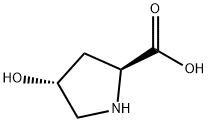
51-35-4

13504-85-3
GENERAL METHOD: To a solution of L-hydroxyproline (78.7 g, 600 mmol) and sodium bicarbonate (126 g, 1.5 mol) in water (900 mL), a solution of benzyl chloroformate (89.6 mL, 630 mmol) in dioxane (90 mL) was slowly added. The reaction mixture was stirred overnight at room temperature. Upon completion of the reaction (monitored by thin-layer chromatography), the reaction mixture was cooled to 0 °C in an ice bath and acidified to pH 2 with 12 M hydrochloric acid (about 75 mL was required). Subsequently, the aqueous phase was extracted with ethyl acetate (3 × 500 mL), the organic phases were combined and washed with saturated brine (3 × 200 mL), dried over anhydrous sodium sulfate, and concentrated under reduced pressure to give a colorless viscous oil. The yield was quantitative, and the product could be directly used in the next reaction without further purification.
Yield:13504-85-3 190 g
Reaction Conditions:
with sodium hydrogencarbonate;sodium carbonate in acetone at 22; for 15 h;Cooling with ice;
Steps:
9.1 Preparation of (254R)-1 -(benzyloxycarbonyl)-4-hydroxypyrrolidine-2-carboxylic acid.
To a 3 L flask were added (25,4R)-4-hydroxypyrrolidine-2-carboxylic acid (87.0 g,663.46 mmol, 1.0 eq.), water (1500 ml), Na2CO3 (141.0 g, 1330 mmol, 2.0 eq.) andNaHCO3 (55.7 g, 663 mmol, 1.0 eq.). Acetone (250 ml) was added to the solution which was then cooled in an ice-water bath. To the mixture was slowly added Cbz-Cl (141.0 g, 829 mmol, 1.25 eq.). After addition, the reaction mixture was warmed gradually to 22 °C and was stirred for 15 h. The reaction mixture was washed with MTBE (600 ml x 2). To the aqueous phase was slowly added aqueous 1 N HCI until pH-2 was achieved. The resulting mixture was extracted with EtOAc (1 L x 3) and the combined organic layer was dried over MgSO4, filtered and concentrated underreduced pressure to provide 190 g of the title compound as an oil, which was taken to the next step without further purification. LCMS: MS = 287.8 (M+H).
References:
WO2018/20358,2018,A1 Location in patent:Page/Page column 84; 85
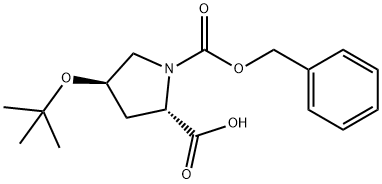
85201-91-8
68 suppliers
$14.00/1g

13504-85-3
287 suppliers
$5.00/1g
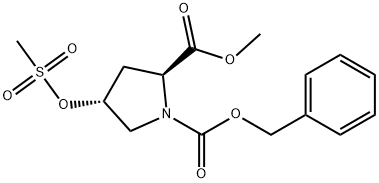
117811-78-6
17 suppliers
$394.31/1g

100-51-6
1434 suppliers
$5.00/100g

13504-85-3
287 suppliers
$5.00/1g
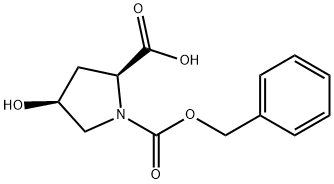
13504-86-4
84 suppliers
$18.00/100mg