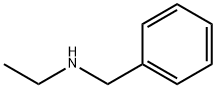
N-Ethylbenzylamine synthesis
- Product Name:N-Ethylbenzylamine
- CAS Number:14321-27-8
- Molecular formula:C9H13N
- Molecular Weight:135.21

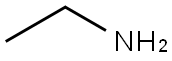
100-52-7
971 suppliers
$20.37/250g
75-04-7
415 suppliers
$10.00/20mg

14321-27-8
234 suppliers
$7.00/5g
Yield:14321-27-8 94.9%
Reaction Conditions:
Stage #1: benzyl aldehyde;ethylamine in methanol at 20;
Stage #2: with sodium tetrahydridoborate in methanol; for 6 h;
Steps:
General procedure for the preparation of compounds 7a-d.
General procedure: To a mixture of benzaldehydes (10 mmol) in methanol (20 mL), the methylamine or ethylamine(10 mmol) was added. The reaction mixturewas stirred at room temperature for 3-4 h, and then sodium borohydride (5.0mmol) was added in batches and the mixture was further stirred for anotherperiod of 6 h. The reaction was then quenched by the addition of water (10 mL),and washed with diethyl ether (20 mL× 3). The combined organic phases werewashed with saturated aqueous NaCl (30 mL), dried over Na2SO4,and filtered. The solvent was evaporated to dryness under reduced pressure. The residue was purified on a silica gel chromatography usingmixtures of CH2Cl2/CH3OH as eluent to obtainthe light yellow oil 7a-d.
References:
Pan, Wanli;Hu, Ke;Bai, Ping;Yu, Lintao;Ma, Qinge;Li, Tao;Zhang, Xu;Chen, Changzhong;Peng, Kelin;Liu, Wenmin;Sang, Zhipei [Bioorganic and Medicinal Chemistry Letters,2016,vol. 26,# 10,p. 2539 - 2543] Location in patent:supporting information
614-17-5
40 suppliers
$172.00/50mg

14321-27-8
234 suppliers
$7.00/5g
588-46-5
142 suppliers
$5.00/100mg

14321-27-8
234 suppliers
$7.00/5g
6852-54-6
13 suppliers
$882.88/5G

14321-27-8
234 suppliers
$7.00/5g
772-54-3
107 suppliers
$10.00/5g

14321-27-8
234 suppliers
$7.00/5g