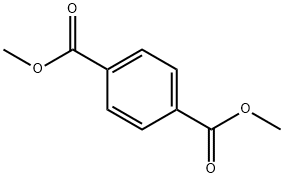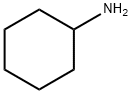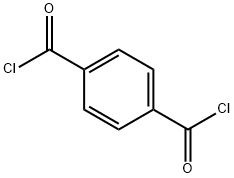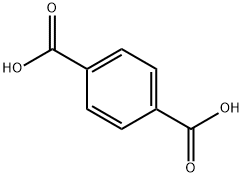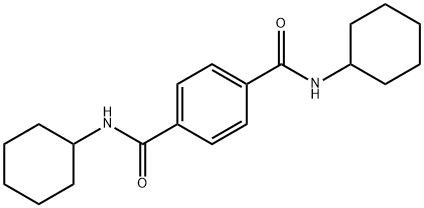
N,N'-DICYCLOHEXYLTEREPHTHALAMIDE synthesis
- Product Name:N,N'-DICYCLOHEXYLTEREPHTHALAMIDE
- CAS Number:15088-29-6
- Molecular formula:C20H28N2O2
- Molecular Weight:328.45
Yield:15088-29-6 88.4%
Reaction Conditions:
with pyridine;potassium carbonate at 135; for 30 h;Temperature;Pressure;Reagent/catalyst;
Steps:
3 Example 3
This example is used to illustrate the preparation of N, N'-dicyclohexyl terephthalamide provided by the present invention, in which the reaction is accelerated with pyridine and potassium carbonate using cyclohexylamine as the entrainer, After the completion of the reaction, the product was separated by the addition of xylene azeotrope.Under normal pressure, 9.42 g of dimethyl terephthalate, 22.52 g of cyclohexylamine, 25 mL of pyridine and 0.69 g of potassium carbonate were added to a 100 mL three-necked round bottom flask equipped with a condenser, a separator, and a stirrer; Heating the oil bath in the control oil bath temperature of 135 , reaction 30h, the separator to collect the distillate distillate;During the course of the reaction, cyclohexylamine was added in six portions and the partial reaction solution was distilled off at an oil bath temperature of 135 ° C, about 21 g each time, and the time between the addition of cyclohexylamine twice hour;After 30 hours of reaction, xylene was added and a portion of the reaction solution was distilled off at a temperature of 140 ° C in an oil bath. The addition of xylene was repeated twice with 50 mL each time, and the time interval between adding xylene twice 5 hours;After completion of the reaction, the solvent was recovered under reduced pressure, and water was added thereto, and the mixture was filtered and washed with water to obtain 14.08 g of N, N'-dicyclohexylterephthalamide in a yield of 88.4% by weight, See the following infrared spectroscopy analysis), the entire preparation process conducive to environmental protection;IR (KBr tablet, cm-1): 3318 (υNH), 3080-3020 (ν-H), 2937,2851,1626 (υ-HNC = O), 1540, 1501, 1447,
References:
CN106316877,2017,A Location in patent:Paragraph 0032; 0033; 0034; 0035; 0036; 0037; 0038-0093