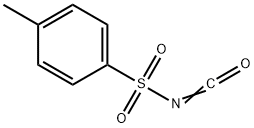
p-Toluenesulfonyl Isocyanate synthesis
- Product Name:p-Toluenesulfonyl Isocyanate
- CAS Number:4083-64-1
- Molecular formula:C8H7NO3S
- Molecular Weight:197.21
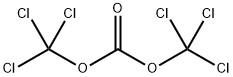
32315-10-9
441 suppliers
$10.00/1g
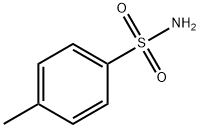
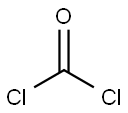
70-55-3
587 suppliers
$6.00/100g

4083-64-1
383 suppliers
$7.00/25g
Yield:4083-64-1 98.33%
Reaction Conditions:
in chlorobenzene at 90 - 105; for 4 h;Temperature;
Steps:
1-6
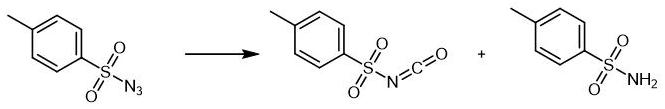
p-toluenesulfonamide 86.04g (content 99.5%, 0.5mol, p-toluenesulfonamide:Dimethyl carbonate = 1:1.1) and 86 g of chlorobenzene were stirred to dissolve, and added to a constant pressure dropping funnel.After the chlorination reaction is completed, the p-toluenesulfonamide chlorobenzene solution is added to the four-necked flask,The temperature was raised to above 90 °C with stirring, and the temperature was controlled at 105 °C for 4 hours.Sampling to detect the complete reaction of the raw materials, connect the condenser tube and the receiving bottle, start to recover the solvent chlorobenzene, control the vacuum degree to -0.08Mpa and the recovery temperature to 105°C.After the solvent chlorobenzene recovery is completed, the material is transferred into the distillation flask,Connect the vacuum pump to evacuate to below -0.095Mpa, heat up and distill,97.73 g of p-toluenesulfonyl isocyanate were obtained, the detected content was 99.19%, and the experimental yield was 98.33%.
References:
CN114181120,2022,A Location in patent:Paragraph 0007; 0015-0038
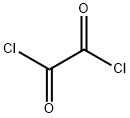
79-37-8
490 suppliers
$17.67/10gm:

70-55-3
587 suppliers
$6.00/100g

4083-64-1
383 suppliers
$7.00/25g
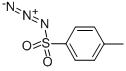
941-55-9
200 suppliers
$75.00/5G
201230-82-2
1 suppliers
inquiry

4083-64-1
383 suppliers
$7.00/25g

70-55-3
587 suppliers
$6.00/100g
![[N-(p-Toluenesulfonyl)imino]phenyliodinane](/CAS/GIF/55962-05-5.gif)