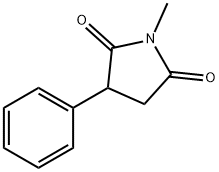
PHENSUXIMIDE synthesis
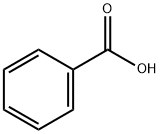
- Product Name:PHENSUXIMIDE
- CAS Number:86-34-0
- Molecular formula:C11H11NO2
- Molecular Weight:189.21
Yield: 92%
Reaction Conditions:
with 4,4'-bipyridine;palladium diacetate;acetic acid in tetrahydrofuran;water at 80; for 24 h;Schlenk technique;Temperature;
Steps:
Typical procedure for conjugate addition of arylboronic acids to N-methylmaleimide:
To a Schlenk tube, phenylboronic acid (244 mg, 2.0 mmol), N-methylmaleimide(111 mg, 1.0 mmol), Pd(OAc)2 (11.2 mg, 0.050 mmol), bpy (31.2 mg,0.20 mmol), HOAc (1.0 mL), THF (2.0 mL) and H2O (0.6 mL) were mixed.The mixture was stirred at 80°C in air for 24 hrs until the reaction completedas monitored by TLC. Water (10mL) was added to the reaction, and the mixture was neutralized with NaHCO3, then extracted with CH2Cl2(10 mL×3), dried by Na2SO4 and concentrated. The residue was purified by flash chromatography (EtOAc: petroleum ether 1: 3) to give 174 mg product 3aa with 92% yield as acolorless oil. N-Methyl-2-phenylsuccinimide3(3aa) Oil.1H NMR (400 MHz, CDCl3): δ 7.46-7.15 (m, 5H), 4.03 (dd, J = 9.2, 4.4 Hz, 1H), 3.21 (dd, J = 18.8, 9.6 Hz, 1H), 3.07 (s, 3H),2.83 (dd, J = 18.8, 4.0 Hz, 1H); 13CNMR (400 MHz, CDCl3): δ 177.76, 176.18, 137.01, 129.08, 127.85,127.30, 45.84, 37.01, 25.10; IR (KBr) 1694 cm-1; MS (ESI) m/z: 212(M + Na+).
References:
Ji, Jiamin;Yang, Zhenyu;Liu, Rui;Ni, Yuxin;Lin, Shaohui;Pan, Qinmin [Tetrahedron Letters,2016,vol. 57,# 25,p. 2723 - 2726] Location in patent:supporting information
853955-69-8
16 suppliers
$39.00/250mg
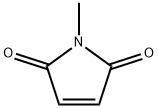
930-88-1
171 suppliers
$6.00/1g

86-34-0
69 suppliers
$29.00/5mg

930-88-1
171 suppliers
$6.00/1g

98-80-6
740 suppliers
$5.00/5g
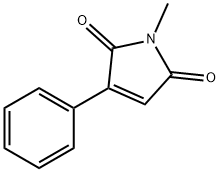
54433-49-7
14 suppliers
inquiry

86-34-0
69 suppliers
$29.00/5mg