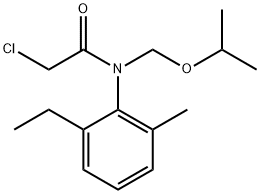
Propisochlor synthesis
- Product Name:Propisochlor
- CAS Number:86763-47-5
- Molecular formula:C15H22ClNO2
- Molecular Weight:283.79
24549-06-2
276 suppliers
$7.00/25g

79-04-9
379 suppliers
$12.00/5g

86763-47-5
101 suppliers
$105.00/50mg
Yield:86763-47-5 98.6%
Reaction Conditions:
Stage #1:6-ethyl-o-toluidine with Methoxyacetone;toluene-4-sulfonic acid in toluene at 88; for 10 h;
Stage #2: with hydrogen at 50; under 56255.6 Torr; for 4.5 h;Autoclave;Inert atmosphere;
Stage #3:chloroacetyl chloride with sodium carbonate in water;toluene at 25; for 0.5 h;Reagent/catalyst;
Steps:
2
First to the reactor (here the reactor is a four-necked flask,In industrial production, it can be replaced with a 400 ml toluene solvent of analytically pure purity by replacing it with a reaction vessel.then,An equal volume of methoxyacetone with toluene solvent and 200 ml of 2,6-methylethylaniline (0.104 mol) and 28 mg of p-toluenesulfonic acid were added to the reaction vessel.Performing an imine synthesis reaction,After the raw materials are added,Turn on the agitation,Warming up to 88 ° C,Control and maintain the temperature of the reaction solution at 88 ° C,The reaction time is 10h,This step is a condensation reaction,Condensation,Means that two or more organic compounds interact with each other,Eventually a covalent bond combines to form a new, larger molecule reaction. In the process of the reaction,Usually one or more molecules of water or some small substances are removed.Such as common hydrogen chloride,Alcohol characteristics, etc.The condensation reaction can occur between molecules.Can also occur inside the molecule,There are also condensation reactions that do not remove any small molecules.Like a common nucleophilic addition reaction,After the condensation reaction between the primary amine and the carbonyl compound,Since the condensation reaction of the amine and the carbonyl compound is a reversible reaction,Therefore, the imine will undergo a hydrolysis reaction under acidic conditions.Will decompose to get the original carbonyl compound and amine,Simultaneously,The nitrogen atom of the condensation product also has a hydrogen atom.So you will lose a molecule of water,Therefore, how to ensure that the water generated by the reaction is continuously removed from the reaction system in time.It is the key to promoting the positive progress of the reaction.By using water-insoluble and azeotropic toluene as a solvent,In the reaction, the reaction water was separated from the reaction system by refluxing water. After the reaction,Vacuum distillation under reduced pressure,Steam the excess solvent,The organic layer is concentrated in vacuo to give the oily product, the imine.The organic layer was concentrated in vacuo to give an oily product of 250 g.The reaction yield was 88.4%. Adding the obtained imine to the autoclave,In this embodiment,In order to facilitate the addition of the catalyst,A total of 300 g of imine was added.And adding 15 ml of a mixture of catalyst 1-S-diphenylphosphino-2-R-bis(3,5-dimethylphenyl)phosphineferrocene-ruthenium and a catalyst ruthenium metal complex,After the feeding is completed,The autoclave was tightened and then replaced with nitrogen and hydrogen for 2 times.After the replacement,Use the purpose of rotation replacement to start stirring.The reaction is at 50 ° C,The reaction was carried out under the reaction conditions of 7.5 MPa for 4.5 hours.Obtaining an amine ether,The reaction of imine catalytic hydrogenation is more common.However, selective catalytic hydrogenation or directed catalytic hydrogenation produces stereoisomerized target products,Especially in the pursuit of high selectivity,High yield,High quality today is very challenging. There are many common reactions involving imines.Hydrolyzed by imine,Producing the corresponding amine and carbonyl compound,Most other reactions involving imines are similar to the chemical reactions of aldehydes with ketone compounds.The imine can also be hydrogenated and reduced.An amine is formed.This step reaction is an asymmetric hydrogenation reduction reaction.It is also a stereoselective response. The prochiral group carbonyl group in the reactant,Double keys, etc.A reduction reaction occurs under the action of a chiral factor to obtain a related amount of a stereoisomer product,These products can be both enantiomers of the enantiomers.It may also be a diastereomer.to this end,The choice of chiral catalyst,Is an important factor in the reaction product,Due to the high viscosity of the imine,to this end,In order to weaken the covalent bond between the two hydrogens of the hydrogen molecule,In this embodiment,Use a base metal complex to work,In order to increase the hydrogen source and the hydride in the active center of the catalyst,To increase the e.e value of the target amine ether,Highly active 1-S-diphenylphosphino-2-R-bis(3,5-dimethylphenyl)phosphinoferrocene-ruthenium was selected as the catalyst.By the combination of two catalysts,The yield of the amine ether is 382 g.The yield is 95%.Here the volume ratio of the two catalysts is 2:1,That is, 1-S-diphenylphosphino-2-R-bis(3,5-dimethylphenyl)phosphine-ferrocene-ruthenium 10 ml,The catalyst rhodium metal complex was 5 ml. 100 g of amine ether was added to the stirred reactor (here in a four-burning flask with mechanical stirring and dropping device).At the same time, adding 220 ml of toluene solvent and 220 ml of water acting solvent,75g of sodium carbonate as a base,High speed mixing,Then slowly add 40ml of material chloroacetyl chloride,The temperature is around 25 °C,After the reaction is completed, add 300 ml of water and stir for 30 minutes.Then let stand layer. The organic layer was dried over anhydrous magnesium sulfate.filter.Concentrated to obtain about 152 g of the purified metolachlor product.Sampling HPLC analysis of ee values,It is 82%.The yield is 98.60%,The purity was 98.2% after being calibrated by liquid chromatography external standard method (quality)fraction),Due to the high viscosity of the amine ether,Poor dispersion in the water phase,therefore,In this embodiment,Using a toluene solvent as a solvent,Conducive to the dispersion of reactants,To promote the reaction,The role of water is to extract the HCl formed in the reaction out of the organic phase.And as a reaction medium for HCl and inorganic alkali sodium carbonate,It also helps to speed up the reaction.
References:
Shandong Wan Hao Feiye Co., Ltd.;Zhang Peng;Yang Xindong;Ding Shaowu;Wu Buhua;Dang Zhao;Xing Chaofeng;Guo Yufeng;Qiu Lihe CN109096137, 2018, A Location in patent:Paragraph 0017-0030