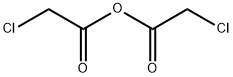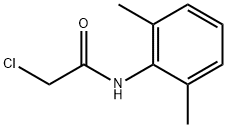
2-Chloro-N-(2,6-dimethylphenyl)acetamide synthesis
- Product Name:2-Chloro-N-(2,6-dimethylphenyl)acetamide
- CAS Number:1131-01-7
- Molecular formula:C10H12ClNO
- Molecular Weight:197.66
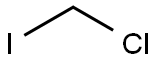
593-71-5
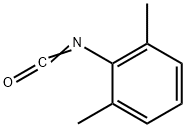
28556-81-2

1131-01-7
General method: Chloroiodomethane (1.5 eq.) is slowly added to a solution of 2,6-dimethylphenyl isocyanate (1.0 eq.) in anhydrous ether (1 M concentration) at -78 °C. After 2 minutes of reaction, a 1.5 M solution of methyl lithium-lithium bromide (1.25 equiv.) ether was added dropwise over 5 minutes. Maintaining this temperature, the reaction mixture was stirred for the indicated time (see Table 1 and Scheme 2 for specific times). Upon completion of the reaction, aqueous ammonium chloride solution (2 mL/mmol substrate) was added, the cooling bath was removed, and stirring was continued until the mixture was brought to room temperature. Subsequently, the reaction mixture was extracted with ether (2 x 5 mL) and the organic phase was washed sequentially with water (5 mL) and brine (10 mL). The organic layer was dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to give pure 2,6-dimethylchloroacetanilide.
87-62-7
464 suppliers
$10.00/5 g

1131-01-7
373 suppliers
$7.00/5g
Yield:1131-01-7 89%
Steps:
289.A 2-chloro-N-(2,6-dimethylphenyl)acetamide
EXAMPLE 289A 2-chloro-N-(2,6-dimethylphenyl)acetamide The procedure described in Example 22A was followed, substituting 2,6-dimethylphenylamine for 3,4,5-trimethoxyaniline to provide the title compound (7.21 g, 89%) 1H NMR (300 MHz, CDCl3) δ 2.25 (s, 6H), 4.26 (s, 2H), 6.65 (t, J=7.46 Hz, 1H, 6.95 (d, J=7.46 Hz, 2H), 7.84 (s, 3H); MS (ESI) m/e 198 (M+H)+.
References:
Bhatia, Pramila A.;Daanen, Jerome F.;Hakeem, Ahmed A.;Kolasa, Teodozyj;Matulenko, Mark A.;Mortell, Kathleen H.;Patel, Meena V.;Stewart, Andrew O.;Wang, Xueqing;Xia, Zhiren;Zhang, Henry Q. US2004/29887, 2004, A1

144-55-8
1413 suppliers
$10.00/25g

79-04-9
381 suppliers
$12.00/5g

87-62-7
464 suppliers
$10.00/5 g

1131-01-7
373 suppliers
$7.00/5g