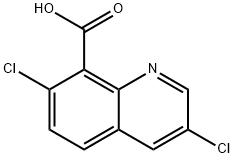
Quinclorac
- CAS No.
- 84087-01-4
- Chemical Name:
- Quinclorac
- Synonyms
- FACET;QUINCHLORAC;dichloroquinolinicacid;3,7-dichloro-8-quinolinecarboxylicaci;3,7-DICHLOROQUINOLINE-8-CARBOXYLIC ACID;Drive;QUEEN;BAS 514;FAS-NOX;Facet LA
- CBNumber:
- CB3265860
- Molecular Formula:
- C10H5Cl2NO2
- Molecular Weight:
- 242.06
- MDL Number:
- MFCD00072495
- MOL File:
- 84087-01-4.mol
| Melting point | 274°C |
|---|---|
| Boiling point | 405.4±40.0 °C(Predicted) |
| Density | 1.7500 |
| refractive index | 1.6100 (estimate) |
| Flash point | 100 °C |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | -3.26±0.10(Predicted) |
| color | Pale Yellow |
| BRN | 7761858 |
| InChIKey | FFSSWMQPCJRCRV-UHFFFAOYSA-N |
| EWG's Food Scores | 1 |
| FDA UNII | 3J06V625EE |
| EPA Substance Registry System | Quinclorac (84087-01-4) |
Quinclorac price More Price(20)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 36521 | Quinchlorac PESTANAL | 84087-01-4 | 250mg | $86.8 | 2024-03-01 | Buy |
| TRC | Q670300 | Quinclorac | 84087-01-4 | 1g | $145 | 2021-12-16 | Buy |
| AK Scientific | J11559 | Quinclorac | 84087-01-4 | 1g | $172 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FD44000 | 3,7-Dichloro-8-quinolinecarboxylic acid | 84087-01-4 | 500mg | $195 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FD44000 | 3,7-Dichloro-8-quinolinecarboxylic acid | 84087-01-4 | 250mg | $122.5 | 2021-12-16 | Buy |
Quinclorac Chemical Properties,Uses,Production
Uses
Quinclorac is a disubstituted quinolinecarboxylic acid that is part of a new class of highly selective auxin herbicides. Quinclorac is used in rice to control dicotyledonous and monocotyledonous weeds, particularly barnyardgrass (Echinochloa crus-galli). Quinclorac is also used for weed control in turfgrasses.
Uses
Quinclorac is a disubstituted quinolinecarboxylic acid that is part of a new class of highly selective auxin herbicides. Quinclorac is used in rice to control dicotyledonous and monocotyledonous weeds , particularly barnyardgrass (Echinochloa crus-galli). Quinclorac is also used for weed control in turfgrasses.
Definition
ChEBI: A quinolinemonocarboxylic acid that is quinoline-8-carboxylic acid in which the hydrogens at positions 3 and 7 have been replaced by chlorines. It is used (particularly as its dimethylamine salt, known as quinclorac-dimethylammonium) as a (rather persisten ) herbicide for the post-emergence control of weeds in rice, grass and turf. It is not approved for use within the European Union.
Pharmacology
The mechanism of action of quinclorac is controversial. Koo et al. (27) reported that quinclorac inhibited the incorporation of glucose into the cell wall of maize root cells. The synthesis of cellulose as well as some hemicellulose was inhibited at concentrations that inhibited whole plant growth, leading to the conclusion that quinclorac inhibits cell expansion by inhibiting glucose incorporation into the cell wall (27). These authors found that quinclorac inhibited root elongation in sensitive grasses at concentrations that inhibited cell wall biosynthesis. Cell wall biosynthesis in tolerant grasses was much less affected by quinclorac compared with sensitive grasses (28). Grossmann, on the other hand, believes that quinclorac acts as an auxinic herbicide in grasses as well as broadleaf plants, and that its herbicidal effects are due to production of cyanide (which is a byproduct of ethylene biosynthesis) and induction of the plant hormone abscisic acid (29). In this scenario, inhibition of cell wall biosynthesis would be considered a side effect, and not the primary cause of herbicidal injury. Differences in tolerance to quinclorac in this case are solely due to differences in ethylene induction, in that tolerant plants do not respond to quinclorac by synthesizing ethylene (29,30).
Metabolism
Quinclorac has been under development by BASF since
1982, and it has been marketed since 1984, as the herbicide
Facet. Synthesis data are not currently available.
Absorption and uptake of quinclorac is rapid, with 85%
uptake in crabgrass within the first 30 minutes.
Although uptake of quinclorac is rapid, translocation is
generally poor, especially in sensitive plants. Tolerant
species, such as Kentucky bluegrass, tend to transport
more of the chemical away from the area of uptake.
This may, in part, explain how tolerant plants are less
affected by quinclorac. Quinclorac is metabolized very
slowly in both sensitive and tolerant grass species. In
leafy spurge, themajor metabolite found 7 days after foliar
application of quinclorac was a pentosylglucose ester.
In susceptible grasses, early symptoms of quinclorac
activity include rapid chlorosis starting at the elongation
zone of newly expanding leaves. This is followed by
more widespread chlorosis and, eventually, necrosis. On
the other hand, quinclorac seems to affect susceptible
broadleaf plants as an auxinic herbicide. Symptoms
in broadleaf plants begin with induction of ethylene
biosynthesis and epinastic bending of shoots and leaves.
This is followed by growth inhibition, chlorosis, wilting,
and, finally, necrosis.
Toxicity evaluation
Quinclorac is nonflammable and noncorrosive, and it is stable in storage for at least 2 years. Quinclorac is classified as general use, with an oral LD50 > 2.61 g/kg in rats (31).
Quinclorac Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7845 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Wuhan Chemwish Technology Co., Ltd | 027-67849912 | sales@chemwish.com | CHINA | 10828 | 58 |
View Lastest Price from Quinclorac manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-12-23 | Quinclorac
84087-01-4
|
US $50.00-1.00 / KG | 1KG | 99% | g-kg-tons, free sample is available | Henan Fengda Chemical Co., Ltd | |
 |
2023-10-24 | Quinclorac
84087-01-4
|
US $20.00-7.00 / kg | 1kg | 99 | 20 tons | Hebei Yanxi Chemical Co., Ltd. | |
 |
2023-07-29 | Quinclorac
84087-01-4
|
US $0.00 / KG | 1KG | 85%, 88%, 96% | 300MT | Weifang Yuanye Biotechnology Co., Ltd |
-

- Quinclorac
84087-01-4
- US $50.00-1.00 / KG
- 99%
- Henan Fengda Chemical Co., Ltd
-

- Quinclorac
84087-01-4
- US $20.00-7.00 / kg
- 99
- Hebei Yanxi Chemical Co., Ltd.
-

- Quinclorac
84087-01-4
- US $0.00 / KG
- 85%, 88%, 96%
- Weifang Yuanye Biotechnology Co., Ltd