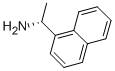
(R)-(+)-1-(1-Naphthyl)ethylamine synthesis
- Product Name:(R)-(+)-1-(1-Naphthyl)ethylamine
- CAS Number:3886-70-2
- Molecular formula:C12H13N
- Molecular Weight:171.24
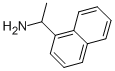
42882-31-5
236 suppliers
$13.00/1g

3886-70-2
501 suppliers
$5.00/1g
Yield:3886-70-2 88.3%
Reaction Conditions:
with p-chlorophenyl pentanoate;hydrogen in toluene at 55; under 75.0075 Torr; for 15 h;Enzymatic reaction;
Steps:
1.1 Preparation of (R) -l- (l-naphthyl) ethylamine:
In a 250 mL three-necked flask, mechanical stirring was slow,Toluene, 4 g of racemic naphthylethylamine, 5.2 g of acyl donor p-chlorophenol valerate, 1 g of Pd / LDH-SA and 0.4 g of lipaseNovozym 435, hydrogen pressure of 0.01MPa, 55 ° C under the conditions of reaction 15h, gas chromatography, Naphthylamine reaction completely, The catalyst (lipase and racemic catalyst) was removed by filtration and used in the next reaction, and the toluene solution was dissolved with sodium hydroxide(R) -amide; and the resulting (R) -amide was stirred at room temperature for 2 hours to remove p-chlorophenol, then dried and evaporated to dryness under reduced pressure to give2mol / L dilute hydrochloric acid hydrolysis 80 ° C 10h, then add ammonia to adjust pH> 10,0.5h after the dichloromethane burning extraction, drying, rotary steamTo give 3.538 (10-1- (1-naphthyl) ethylamine in a yield of 88.3%.
References:
CN104592037,2016,B Location in patent:Paragraph 0040
![Phosphinic amide, N-[(1R)-1-(1-naphthalenyl)ethyl]-P,P-diphenyl-](/CAS/20210305/GIF/23943-58-0.gif)
23943-58-0
0 suppliers
inquiry

3886-70-2
501 suppliers
$5.00/1g
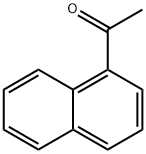
941-98-0
376 suppliers
$5.00/10g

3886-70-2
501 suppliers
$5.00/1g

42882-31-5
236 suppliers
$13.00/1g
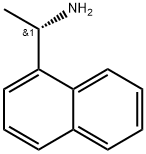
10420-89-0
325 suppliers
$6.00/1g

3886-70-2
501 suppliers
$5.00/1g