ethyl 2-chloro-4-ethoxy-nicotinate synthesis
- Product Name:ethyl 2-chloro-4-ethoxy-nicotinate
- CAS Number:127423-61-4
- Molecular formula:C10H19NO5S
- Molecular Weight:265.33
83220-73-9

124-63-0

127423-61-4
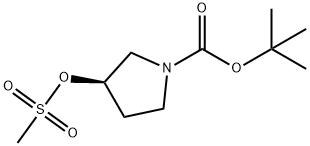
General Steps: Step 1: Synthesis of (R)-1-(tert-butoxycarbonyl)pyrrolidin-3-yl methanesulfonate. A solution of tert-butyl (R)-3-hydroxypyrrolidine-1-carboxylate (1.0 g, 5.3 mmol) and triethylamine (2.77 mL, 20 mmol) was cooled in an ice water bath in dichloromethane (20 mL). To this solution was added methosulfonyl chloride (1.15 mL, 15 mmol) diluted in dichloromethane (10 mL) dropwise. The reaction mixture was stirred at room temperature for 12 hours. Upon completion of the reaction, water was added and the organic phase was extracted with dichloromethane (3 x 50 mL). The combined organic phases were dried and purified by silica gel chromatography (50% ethyl acetate:hexane to 100% ethyl acetate gradient) to afford (R)-1-(tert-butoxycarbonyl)pyrrolidin-3-yl methanesulfonate (1.53 g, brown oil, 100% yield).
109431-87-0
363 suppliers
$5.00/1g

124-63-0
0 suppliers
$10.00/5g

127423-61-4
64 suppliers
$15.00/100mg
Yield: 100%
Reaction Conditions:
with triethylamine in dichloromethane at 20;Cooling with ice;
Steps:
20.1
Example 20. N-(4-(4-amino-l-((S)-pyrrolidin-3-yl)-lH-pyrazolo[3,4-d]pyrimidin-3- yl)phenyl)-3-(trifluoromethyl)benzamide (AD070); Step 1; [0200] (i?)-l-(ter£-butoxycarbonyl)pyrrolidin-3-yl methanesulfonate. A solution of (R)- tert-bntyl 3-hydroxypyrrolidine-l-carboxylate (1.0 g, 5.3 mmol) and triethylamine (2.77 mL, 20 mmol) in CH2Cl2 (20 mL) was cooled in an ice-water bath. To this, methanesulfonyl chloride (1.15 mL, 15 mmol) diluted in CH2Cl2 (10 mL) was added dropwise. The reaction was left stirring for 12 hours at room temperature. Water was added, and organic phases extracted in CH2Cl2 (3x 50 mL), which were subsequently dried onto silica and purified by silica gel chromatography (50% EtOAc:Hexanes to 100% EtOAc gradient) to afford (R)-I- (te/t-butoxycarbonyl)pyrrolidin-3-yl methanesulfonate (1.53 g, brown oil, 100% yield).
References:
THE REGENTS OF THE UNIVERSITY OF CALIFORNIA;DAR, Arvin;SHOKAT, Kevan M. WO2010/45542, 2010, A2 Location in patent:Page/Page column 73

124-63-0
0 suppliers
$10.00/5g
101469-92-5
360 suppliers
$6.00/5g

127423-61-4
64 suppliers
$15.00/100mg

109431-87-0
363 suppliers
$5.00/1g

124-63-0
0 suppliers
$10.00/5g
121-44-8
906 suppliers
$9.00/5g
554-68-7
504 suppliers
$6.00/25g

127423-61-4
64 suppliers
$15.00/100mg

24424-99-5
868 suppliers
$13.50/25G

127423-61-4
64 suppliers
$15.00/100mg
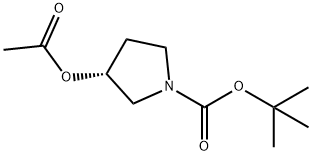
101408-94-0
1 suppliers
inquiry

127423-61-4
64 suppliers
$15.00/100mg