Salidroside synthesis
- Product Name:Salidroside
- CAS Number:10338-51-9
- Molecular formula:C14H20O7
- Molecular Weight:300.3

![β-D-Glucopyranoside, 2-[4-(phenylmethoxy)phenyl]ethyl](/CAS/20210305/GIF/183209-56-5.gif)
183209-56-5
0 suppliers
inquiry

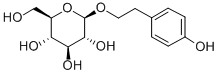
10338-51-9
535 suppliers
$5.00/5mg
Yield:10338-51-9 93%
Reaction Conditions:
with 5%-palladium/activated carbon;hydrogen in methanol at 20;Darkness;Green chemistry;
Steps:
15 Example 15 Preparation of 2-(4-hydroxyphenyl)ethyl-β-D-glucopyranoside (resorpirin)
1.1 g (2.8 mmol) of 1-O-(4-benzyloxy)-phenylethyl yl -β-D- glucopyranoside and 5% Pd / C 0.1 g of catalyst, and then repeatedly evacuated air charge of hydrogengas 3-5 times, and 20 mL of anhydrous methanol, protected from light, stirred at room temperature under a hydrogen atmosphere overnight.Filtration, concentration to dryness under reduced pressure,and recrystallized from ethanol to give 1-(4-hydroxy)-phenethyl-β-D-s-glucopyranoside as a white powder, which is aweight of 0.78 g. The yield was 93%.
References:
CN107880085,2018,A Location in patent:Paragraph 0039
28251-63-0
3 suppliers
inquiry

10338-51-9
535 suppliers
$5.00/5mg
![β-D-Glucopyranoside, 2-[4-(acetyloxy)phenyl]ethyl, 2,3,4,6-tetraacetate](/CAS/20210305/GIF/39032-08-1.gif)
39032-08-1
3 suppliers
inquiry

10338-51-9
535 suppliers
$5.00/5mg

1346265-60-8
0 suppliers
inquiry

10338-51-9
535 suppliers
$5.00/5mg
![4-[2-(β-D-Glucopyranosyloxy)ethyl]-4-hydroxy-2,5-cyclohexadien-1-one](/CAS/20210111/GIF/40661-45-8.gif)
40661-45-8
30 suppliers
inquiry

10338-51-9
535 suppliers
$5.00/5mg

101489-37-6
0 suppliers
inquiry
![Cyclohexanone, 4-[2-(β-D-glucopyranosyloxy)ethyl]-4-hydroxy-](/CAS/20210305/GIF/123563-44-0.gif)
123563-44-0
0 suppliers
inquiry