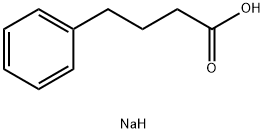
Sodium 4-phenylbutyrate synthesis
- Product Name:Sodium 4-phenylbutyrate
- CAS Number:1716-12-7
- Molecular formula:C10H13NaO2
- Molecular Weight:188.2
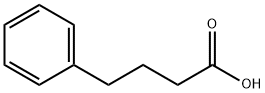
1821-12-1

1716-12-7
0.40 g (2.436 mmol) of 4-phenylbutyric acid was suspended in 30 mL of water, followed by the addition of 0.097 g (2.436 mmol) of sodium hydroxide. After complete dissolution of the acid, the reaction solution was concentrated by rotary evaporator and azeotropically dehydrated twice with toluene. Finally, 0.44 g (96.0% yield) of solid sodium 4-phenylbutyrate was obtained.

1821-12-1
421 suppliers
$6.00/5g

1716-12-7
298 suppliers
$9.00/250mg
Yield:1716-12-7 96%
Reaction Conditions:
with sodium hydroxide in water
Steps:
0.40 g (2.436 mmol) of 4-phenylbutyric acid are suspended in 30 ml of H2O, and then 0.097 g (2.436 mmol) of sodium hydroxide is added. After the acid has been dissolved, the solution is concentrated by rotary evaporation and twice dewatered azeotropically with toluene. This gives 0.44 g (96.0%) of sodium 4-phenylbutyrate in solid form.
References:
BAYER CROPSCIENCE AG US2012/77677, 2012, A1 Location in patent:Page/Page column 3
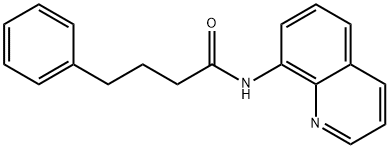
1449300-08-6
0 suppliers
inquiry

1716-12-7
298 suppliers
$9.00/250mg

108-86-1
524 suppliers
$10.00/5g

1716-12-7
298 suppliers
$9.00/250mg
578-66-5
326 suppliers
$6.00/1g

1716-12-7
298 suppliers
$9.00/250mg