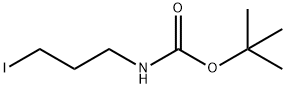
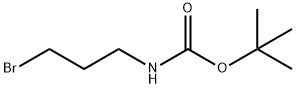
TERT-BUTYL (3-IODOPROPYL)CARBAMATE synthesis
- Product Name:TERT-BUTYL (3-IODOPROPYL)CARBAMATE
- CAS Number:167479-01-8
- Molecular formula:C8H16INO2
- Molecular Weight:285.12
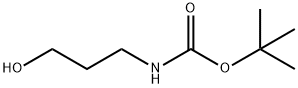
58885-58-8

167479-01-8
General procedure for the synthesis of tert-butyl (3-iodopropyl) carbamate from tert-butyl N-(3-hydroxypropyl) carbamate: firstly, 3-amino-1-propanol (3.22 mL, 40 mmol) was dissolved in 50 mL of dichloromethane (CH2Cl2), triethylamine (Et3N, 5.6 mL, 40 mmol) was added, and stirred for 30 min. Subsequently, a solution of pre-dissolved di-tert-butyl dicarbonate (Boc2O, 9.6 g, 44 mmol) in 50 mL of CH2Cl2 was slowly added and the reaction mixture was stirred at room temperature for 14 hours. After completion of the reaction, saturated aqueous ammonium chloride solution (30 mL) was added and the mixture was extracted with CH2Cl2 (3 x 20 mL). The organic phases were combined, dried and concentrated to give the crude product (6.00 g, 86% yield), which was used directly in the next step of the reaction. Next, 1H-imidazole (1.4 g, 20.57 mmol) and triphenylphosphine (5.39 g, 20.57 mmol) were dissolved in 100 mL of CH2Cl2 and cooled to 0 °C before adding iodine (I2, 5.22 g, 20.57 mmol) in small portions. Then, a solution of the crude product (3.0 g, 17.14 mmol) obtained in the previous step dissolved in 20 mL CH2Cl2 was added. The reaction mixture was stirred for 3 h at room temperature and then poured into water (100 mL) and extracted with CH2Cl2 (50 mL). The organic phase was washed sequentially with water, 10% hydrochloric acid solution (12 mL), then extracted with CH2Cl2 (3 x 50 mL), dried and concentrated. Finally, the target product tert-butyl (3-iodopropyl)carbamate (3.78 g, 78% yield) was purified by fast chromatography (silica gel, 15% ethyl acetate/petroleum ether) as a light yellow oil.

58885-58-8
196 suppliers
$10.00/1g
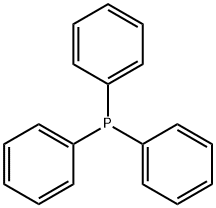
603-35-0
732 suppliers
$5.00/5 g
791-28-6
536 suppliers
$6.00/25g

167479-01-8
54 suppliers
$65.00/250mg
Yield:-
Reaction Conditions:
with 1H-imidazole;iodine in diethyl ether;acetonitrile at 0 - 20;
Steps:
4.B
A mixture of tert-bntyl 3-hydroxypropylcarbamate (19.36 g, 110.5 mmol), triphenylphosphine (34.76 g, 132.6 mmol), imidazole (10.53 g, 154.7 mmol), diethyl ether EPO
References:
3M INNOVATIVE PROPERTIES COMPANY WO2006/86449, 2006, A2 Location in patent:Page/Page column 88-89

58885-58-8
196 suppliers
$10.00/1g

167479-01-8
54 suppliers
$65.00/250mg
83948-53-2
210 suppliers
$6.00/1g

167479-01-8
54 suppliers
$65.00/250mg

288-32-4
1046 suppliers
$5.00/25g

58885-58-8
196 suppliers
$10.00/1g

167479-01-8
54 suppliers
$65.00/250mg

24424-99-5
868 suppliers
$13.50/25G

167479-01-8
54 suppliers
$65.00/250mg