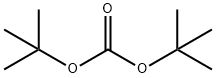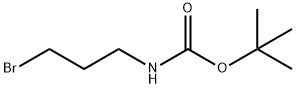
tert-Butyl 3-bromopropylcarbamate synthesis
- Product Name:tert-Butyl 3-bromopropylcarbamate
- CAS Number:83948-53-2
- Molecular formula:C8H16BrNO2
- Molecular Weight:238.12

24424-99-5

5003-71-4

83948-53-2
The general procedure for the synthesis of N-Boc-3-aminopropyl bromide from di-tert-butyl dicarbonate and 3-bromopropylamine hydrobromide is as follows: refer to Example 4, Synthesis of Boc-Aminopropyl Bromide: 1.222 g (5.58 mmol) of 3-bromopropylamine hydrobromide was dissolved in 20 mL of dichloromethane to which was added under cooling in an ice bath 0.778 mL (5.58 mmol) of triethylamine and 50 mL of dichloromethane. A solution of 1.214 g (5.56 mmol) of di-tert-butyl dicarbonate (Boc2O) was added slowly and dropwise over a period of 10 min, followed by stirring of the reaction mixture. After continued stirring at room temperature for 50 min, ethyl acetate was added to the reaction system and the organic phase was washed sequentially with 5% aqueous citric acid, water and saturated saline. The organic layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure to remove the solvent to give 1.304 g of N-Boc-3-aminopropyl bromide in 98% yield. The structure of the product was confirmed by 1H-NMR. 1H-NMR (500 MHz, CDCl3) δ (ppm) = 1.44 (9H, s, Boc group), 2.05 (2H, quintet, -NHCH2CH2CH2Br), 3.28 (2H, quartet, -NHCH2CH2CH2Br), 3.44 ( 2H, triplet, -NHCH2CH2CH2Br), 4.64 (1H, s, NH).

24424-99-5
869 suppliers
$13.50/25G

5003-71-4
380 suppliers
$5.00/10g

83948-53-2
210 suppliers
$6.00/1g
Yield:83948-53-2 98%
Reaction Conditions:
with triethylamine in dichloromethane;ethyl acetate
Steps:
R.4 Synthesis of Boc-aminopropyl bromide:
Reference Example 4 Synthesis of Boc-aminopropyl bromide: In 20 ml of dichloromethane, 1.222 g (5.58 mmol) of 3-bromopropylamine hydrobromide was dissolved, 0.778 ml (5.58 mmol) of triethylamine was added thereto under ice-cooling, and 50 ml dichloromethane solution of 1.214 g (5.56 mmol) of Boc2O was further added dropwise thereto in 10 minutes, followed by stirring. After stirring at room temperature for 50 minutes, ethyl acetate was added thereto, followed by separation by washing with 5% aqueous citric acid solution, water and saturated brine consecutively. After dehydration with sodium sulfate, the solvent was evaporated under reduced pressure to give 1.304 of the titled compound (98%). The structure was identified by 1H-NMR. 1H-NMR (500 MHz,CDCl3) δ (ppm) = 1.44 (9H, s, Boc), 2.05 (2H, quant, -NHCH2CH2CH2Br), 3.28 (2H, q, -NHCH2CH2CH2Br), 3.44 (2H, t, -NHCH2CH2CH2Br), 4.64 (1H, s, NH)
References:
SEIKAGAKU CORPORATION EP1710257, 2006, A1
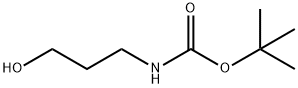
58885-58-8
195 suppliers
$10.00/1g

83948-53-2
210 suppliers
$6.00/1g

18370-81-5
15 suppliers
inquiry

24424-99-5
869 suppliers
$13.50/25G

83948-53-2
210 suppliers
$6.00/1g

24424-99-5
869 suppliers
$13.50/25G

5003-71-4
380 suppliers
$5.00/10g
497-19-8
1488 suppliers
$13.00/300G

83948-53-2
210 suppliers
$6.00/1g