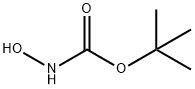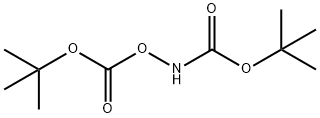
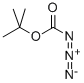
TERT-BUTYL N-(TERT-BUTOXYCARBONYLOXY)CARBAMATE synthesis
- Product Name:TERT-BUTYL N-(TERT-BUTOXYCARBONYLOXY)CARBAMATE
- CAS Number:85006-25-3
- Molecular formula:C10H19NO5
- Molecular Weight:233.26

24424-99-5

85006-25-3
The general procedure for the synthesis of N,O-di-BOC-hydroxylamine from di-tert-butyl dicarbonate was as follows: first, hydroxylamine hydrochloride (550.0 g, 7.9 mol) was dissolved in a solvent mixture of water (5.5 L) and heptane/MTBE (5:1, 5.5 L), and the mixture was cooled to -5 °C. Subsequently, a solution of di-tert-butyl dicarbonate (3.55 Kg, 16.3 mol) and triethylamine (1.67 Kg, 16.5 mol) in heptane/MTBE (5:1, 1.1 L), which was pre-cooled to -5°C, was slowly added dropwise to the above mixture, and the dropwise process lasted for more than 2 hours. The reaction mixture was continued to be stirred at -5°C for 1 hour, then gradually warmed up to room temperature and stirred overnight. Upon completion of the reaction, the organic layer and the aqueous layer were separated, and the organic layer was washed twice with saturated aqueous ammonium chloride solution (2 L) and saturated aqueous sodium chloride solution (1 L) sequentially, then dried with anhydrous sodium sulfate, filtered and concentrated to obtain an oily product, which was converted to a white solid by crystallization. Finally, the resulting solid was stirred with heptane (1 L) in an ice-water bath and filtered to afford the target product N,O-dibOC-hydroxylamine (1360.2 g, 73% yield). The structure of the product was confirmed by 1H NMR (d6-DMSO) with characteristic peaks located at δ 10.7 (broad single peak, 1H), 1.44 (single peak, 9H), 1.40 (single peak, 9H).

24424-99-5
869 suppliers
$13.50/25G
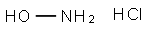
5470-11-1
817 suppliers
$10.00/5 g

85006-25-3
110 suppliers
$25.00/5g
Yield:-
Reaction Conditions:
with triethylamine in tert-butyl methyl ether;water;Petroleum ether
Steps:
1 EXAMPLE 1 : Synthesis of Compounds
N,0-?zs(t-butoxycarbonyl)- hydroxylamine6 was obtained through N,0 diBoc protection of hydroxylamine hydrochloride (di-t-butyl dicarbonate, triethylamine, petroleum ether, t-butyl methyl ether, water).
References:
JOHNS HOPKINS UNIVERSITY;GUTHRIE, Daryl A. WO2013/59194, 2013, A1 Location in patent:Page/Page column 38

24424-99-5
869 suppliers
$13.50/25G

85006-25-3
110 suppliers
$25.00/5g
870-46-2
547 suppliers
$5.00/10g

85006-25-3
110 suppliers
$25.00/5g
1070-19-5
12 suppliers
inquiry

85006-25-3
110 suppliers
$25.00/5g