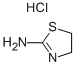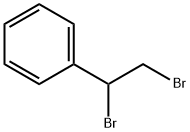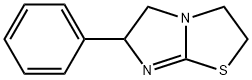
tetramisole synthesis
- Product Name:tetramisole
- CAS Number:5036-02-2
- Molecular formula:C11H12N2S
- Molecular Weight:204.29
Yield:5036-02-2 87.54%
Reaction Conditions:
Stage #1: 2-amino-4,5-dihydrothiazole hydrochloride;(1,2-dibromoethyl)benzenewith sodium carbonate in N,N-dimethyl-formamide at 85; for 2 h;
Stage #2: in N,N-dimethyl-formamide at 160; for 16 h;Temperature;Reagent/catalyst;
Steps:
1-5 Example 1:
(1) FeedingTake 26.43g (0.1mol) of 1,2-dibromoethylbenzene, add it to 100mL DMF solution, and dissolve completely. Add 15.14g (0.11mol) of 2-aminothiazoline hydrochloride, and add 11.67g (0.11mol) of sodium carbonate particles for mixing reaction.(2) The first stage reactionIn the first stage of the reaction, the temperature was raised to 85°C and reacted for 2 hours;(3) The second stage reactionThen, in the second stage of the reaction, 5.84g (0.055mol) of sodium carbonate particles were continuously added, the temperature was raised to 160°C and refluxed, and the reaction was carried out for 16h until the conversion of 1,2-dibromoethylbenzene raw material was completed.(4) Post-processingThe reaction system was cooled to room temperature, filtered and the insoluble matter was discarded, the filtrate was distilled under reduced pressure, DMF was recovered, the solid was concentrated to dryness (19.01g), mixed with 100mL of dichloromethane and 100mL of purified water for stirring and dissolution, and then left to stand for fractionation. Discard the water layer after the layer. Add 100 mL of pure water and mix with the dichloromethane layer obtained above, turn on the stirring, adjust the pH of the mixed solution to 2 with hydrochloric acid, after mixing uniformly, stand still for layering, and discard the dichloromethane layer. Concentrate the obtained water layer to dryness, then add 40 mL of ethanol and stir to disperse the solids, filter out the solids, and dry to obtain 21.25 g of white powder, which is a tetraimidazole hydrochloride product, with a purity of 99.20% and a calculated yield of 87.54%.
References:
CN112358490,2021,A Location in patent:Paragraph 0026-0044
5086-74-8
407 suppliers
$5.00/1g

5036-02-2
34 suppliers
inquiry
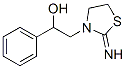
10060-88-5
7 suppliers
inquiry

5036-02-2
34 suppliers
inquiry
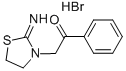
4335-26-6
10 suppliers
$350.00/1g

5036-02-2
34 suppliers
inquiry
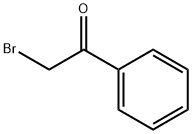
70-11-1
294 suppliers
$10.00/25g

5036-02-2
34 suppliers
inquiry