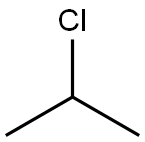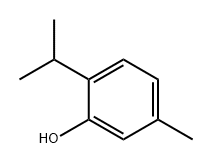
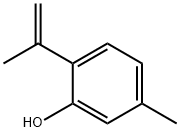
Thymol synthesis
- Product Name:Thymol
- CAS Number:89-83-8
- Molecular formula:C10H14O
- Molecular Weight:150.22
18612-99-2
5 suppliers
inquiry

89-83-8
750 suppliers
$5.00/10g
Yield:89-83-8 98 kg
Reaction Conditions:
with palladium 10% on activated carbon;ammonium formate in ethanol;n-heptane at 20; for 6 h;Reflux;
Steps:
3.3; 3.4
Step 3: Palladium carbon 10% (14 kg, wet weight), ammonium formate (90 kg), and ethanol (130 kg) were added to the reduction kettle, and the reaction was stirred at room temperature for 3 hours. The reaction solution was filtered to remove the catalyst. The filtrate was added with purified water (300 kg), stirred for 15 min, and allowed to stand for 15 min to separate the organic phase. 400 kg of saturated brine was added to the organic phase, and the mixture was stirred for 15 minutes, and the mixture was allowed to stand for 15 minutes, and the organic phase was separated and introduced into a distillation pot. The external temperature is ≤90 ° C, concentrated under reduced pressure to remove n-heptane; the steam is heated by distillation to distill the residue under reduced pressure: a fraction of 110-125 ° C is received, and a total of 130 kg of a light yellow irritating odor liquid is collected.Step 4: A distillation fraction (130 kg) was added to the reduction vessel, n-heptane (450 kg), ethanol (60 kg) was added, and palladium carbon 10% (6 kg, wet weight) and ammonium formate (40 kg) were continuously added. The temperature was slowly raised to reflux, maintained at reflux for 1 h, and then cooled to 20-25 ° C, and ammonium formate (15 kg) was further added. The reaction was incubated for 2 h, and the reaction solution was filtered to remove the catalyst. The filtrate was added with purified water (150 kg), stirred for 15 min, and left to stand for 30 min to separate the organic phase; the organic phase was added with saturated brine (150 kg), stirred for 15 min, and allowed to stand for 15 min. The organic phase is separated.Anhydrous sodium sulfate (50 kg) was added to the organic phase and stirred and dried for 1 h. The desiccant was removed by filtration, and the filtrate was introduced into a distillation pot, and concentrated under reduced pressure to remove the organic phase. To the residue was added n-heptane (130 kg). The temperature was controlled at -5 ° C to 5 ° C for 4 h. After centrifugation, the mother liquor rinses the solids in the kettle. The filter cake was vacuum dried at 30 to 40 ° C for 4 to 6 hours (-0.07 to -0.1 Mpa). A white to off-white crystalline solid of 110 kg was obtained.The obtained crude product (110.00 kg) and n-heptane (130 kg) were placed in a reaction vessel, dissolved by heating, and the filtrate was passed through a membrane and introduced into a crystallizing vessel in a clean zone. The temperature was controlled at 0 to 5 ° C, and the crystallization was carried out for 4 hours. The material was discharged, centrifuged, and the amount of n-heptane (pre-cooled, about -5 to 5 ° C) was used to rinse the filter cake. The filter cake was introduced into a double cone and vacuum-dried at 30 to 40 ° C for 4 to 6 hours (-0.07 to -0.1 Mpa). The material was obtained, and 98.0 kg of a white crystalline solid was obtained. The purity is 99.86%, the melting point is mp: 49 to 51 ° C, and the nonvolatile content is less than 0.05% at 105 ° C. The total reaction yield was 47.03%.The results of the nuclear magnetic resonance spectrum test are as follows, and it can be confirmed as thymol.
References:
Zhangzhou Narcissus Pharmaceutical Co., Ltd.;Beijing Xinkaiyuan Pharmaceutical Technology Co., Ltd.;Yu Kai;Jiang Yugang;Wang Yongguang;Ge Zhimin;Li Zhixiang;Lin Congming CN108911951, 2018, A Location in patent:Paragraph 0059-0062; 0067-0070; 0072; 0075-0078; 0083-0086
108-39-4
507 suppliers
$13.00/25g
67-63-0
1637 suppliers
$16.00/25ML

89-83-8
750 suppliers
$5.00/10g
187737-37-7
1 suppliers
inquiry

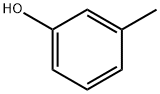
108-39-4
507 suppliers
$13.00/25g
3228-02-2
285 suppliers
$8.00/5g

89-83-8
750 suppliers
$5.00/10g