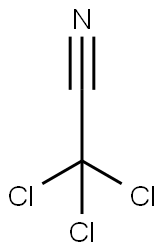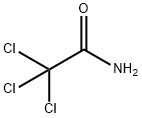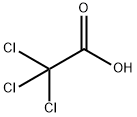
Trichloroacetic acid synthesis
- Product Name:Trichloroacetic acid
- CAS Number:76-03-9
- Molecular formula:C2HCl3O2
- Molecular Weight:163.39
Yield:594-65-0 52% ,76-03-9 52%
Reaction Conditions:
with water at 140; for 6 h;Sealed tube;
Steps:
2.3 General Reaction Procedure
General procedure: In a typical reaction procedure 1 mmol benzonitrile and3 ml of H2O was taken in a sealed tube. About 80 mg ofcatalyst was added to this reaction mixture and stirred at140 °C on a oil bath for stipulated reaction time. Theprogress of the reaction was monitored by using thin layerchromatography. The products were identified by GC-MS(SHIMADZU-2010) analysis by separating the products ona DB-5 column.
References:
Srinivasa Rao;Srivani;Dhana Lakshmi;Lingaiah [Catalysis Letters,2016,vol. 146,# 10,p. 2025 - 2031]
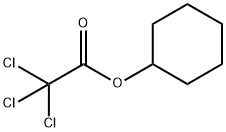
40410-64-8
0 suppliers
inquiry

76-03-9
440 suppliers
$14.00/25g
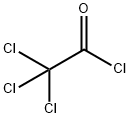
76-02-8
256 suppliers
$38.29/25G

76-03-9
440 suppliers
$14.00/25g
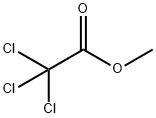
598-99-2
167 suppliers
$21.21/10gm:

76-03-9
440 suppliers
$14.00/25g