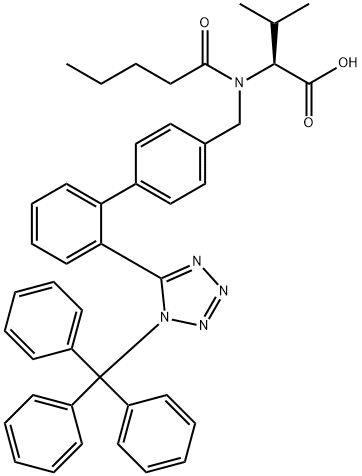
Trityl Valsartan synthesis
- Product Name:Trityl Valsartan
- CAS Number:783369-52-8
- Molecular formula:C43H43N5O3
- Molecular Weight:677.83
![N-[(2′-(1-Triphenylmethyl-Tetrazole-5-Yl)Biphenyl-4-Yl]-Methyl]-N-Valeryl-L- Valine Benzyl Ester](/CAS/20200611/GIF/137864-44-9.gif)
137864-44-9
3 suppliers
inquiry

783369-52-8
9 suppliers
inquiry
Yield:783369-52-8 86%
Reaction Conditions:
with hydrogen;5%-palladium/activated carbon in ethyl acetate at 20; for 3 h;
Steps:
28
A mixture of benzyl N-pentanoyl-N-[{2'-[1-(triphenylmethyl)-1 H-tetrazol-5-yl]- 1 ,1'-biphenyl-4-yl}methyl]-L-valinate (0.3 g) in AcOEt (3 ml_) containing 5% palladium on activated Charcoal (Pd/C, 34 mg ) was hydrogenated at room temperature for 3h. The resulting crude product was filtered through a Celite pad and the solvent was evaporated to dryness to furnish the desired product (0.23 g, 86%). 1H-NMR (400 MHz, CDCI3): δ 0.85 and 0.98 (2 d, 3 H each, J = 6.8 Hz, 2 CH3 (iPr)), 0.88 (t, 3 H, J = 7.2 Hz, CH3 (pentanoyl)), 1.29 (m, 2 H, CH2Me), 1.61 (m, 2 H, CH2Et), 2.36 (m, 2 H, CH2CO), 2.74 (m, 1 H, CH(iPr)), 3.51 (d, 1 H, J = 10.8 Hz, CHN), 4.28 and 4.60 (2 d, 1 H each, J = 16.2 Hz, CH2-N), 6.94-7.92 (m, 23 H, H-Ar) ppm. 13C-NMR (100 MHz, CDCI3): δ 13.8, EPO
References:
WO2006/67216,2006,A2 Location in patent:Page/Page column 27-28
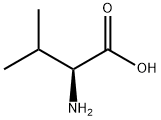
72-18-4
816 suppliers
$5.00/25g

783369-52-8
9 suppliers
inquiry
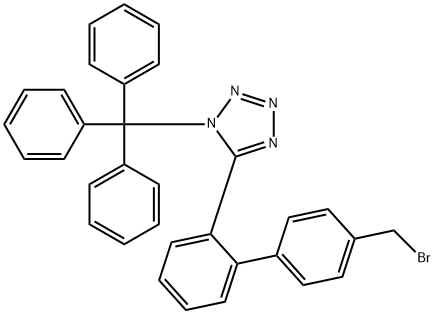
124750-51-2
454 suppliers
$6.00/1g

783369-52-8
9 suppliers
inquiry