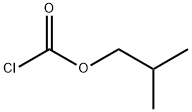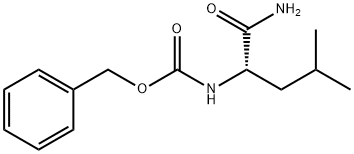
Z-LEU-NH2 synthesis
- Product Name:Z-LEU-NH2
- CAS Number:4801-79-0
- Molecular formula:C14H20N2O3
- Molecular Weight:264.32
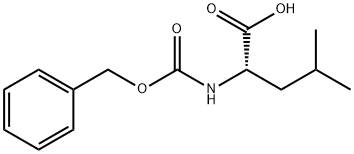
2018-66-8

4801-79-0
General procedure for the synthesis of tert-butyl (S)-(1-amino-4-methyl-1-oxopentan-2-yl)carbamate from (S)-2-(((benzyloxy)carbonyl)amino)-4-methylpentanoic acid: to a colorless solution of 150 mg (0.50 mmol) of (S)-2-(((benzyloxy)carbonyl)amino)-4-methylpentanoic acid in 10 mL of THF was added 67 μL (0.70 mmol, 1.4 eq.) of ethyl chloroformate and 209 μL (1.5 mmol, 3.0 eq.) of triethylamine, and the reaction was carried out at 0 °C. After stirring at 0 °C for 30 min, 0.75 mL of 1.0 M aqueous ammonium chloride (0.75 mmol, 1.5 eq.) was added to the colorless suspension. The mixture was continued to be stirred at 0 °C for 30 min, followed by the addition of 5 mL of water. The colorless clear solution was extracted with 30 mL of ethyl acetate and the aqueous layer was extracted with another 20 mL of ethyl acetate. The organic layers were combined, washed with 5 mL of brine and dried with anhydrous magnesium sulfate. The crude product was purified by silica gel column chromatography using ethyl acetate as eluent to give 129 mg (86% yield) of tert-butyl (S)-(1-amino-4-methyl-1-oxopentan-2-yl)carbamate. The product is a colorless powder with a melting point of 123-125 °C; specific spin [α]27D = -11.2 (c 1.00, methanol); 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) data are consistent with the structure; infrared spectroscopy (KBr) shows characteristic absorption peaks; high-resolution mass spectrometry (ESI-TOF) measured the molecular ion peaks were consistent with the calculated values; the enantiomeric purity was determined by chiral HPLC (Chiralcel AD column, hexane/2-propanol = 90/10) with a retention time of 10.7 min.
Yield:4801-79-0 100%
Reaction Conditions:
with 4-methyl-morpholine;ammonia in tetrahydrofuran;ethyl acetate;
Steps:
173.a a)
a) N-benzyloxycarbonyl-L-leucinamide To a stirring solution of N-benzyloxycarbonyl-L-leucine (4.6 g, 17.3 mmol) in THF, cooled to -40° C., was added N-methylmorpholine (3.68 g, 36.4 mmol; 4.0 mL) and isobutyl chloroformate (2.37 g, 17.3 mmol; 2.25 mL). After stirring for 15 min, ammonia was bubbled through the solution for 5 min. The solution was warmed to room temperature, evaporated, and the residue was dissolved in ethyl acetate, washed with 0.1 N Hcl, and saturated brine, then dried (MgSO4), filtered and evaporated to dryness to give the title compound as a white solid (4.58 g, 100%).
References:
US2002/49316,2002,A1

2018-66-8
351 suppliers
$8.00/5g

4801-79-0
40 suppliers
$9.00/1g
![L-Leucine, N-[N-[N-[N-[N-[N-[N-[(phenylmethoxy)carbonyl]-L-leucyl]-L-seryl]-L-seryl]-L-leucyl]-L-leucyl]-L-seryl]-, methyl ester](/CAS/20210305/GIF/158011-13-3.gif)
158011-13-3
0 suppliers
inquiry

4801-79-0
40 suppliers
$9.00/1g
51021-87-5
60 suppliers
$16.80/1G

4801-79-0
40 suppliers
$9.00/1g
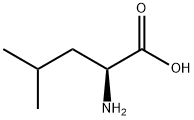
61-90-5
857 suppliers
$10.00/25G

4801-79-0
40 suppliers
$9.00/1g