N,N-Dimethylformamid Chemische Eigenschaften,Einsatz,Produktion Methoden
ERSCHEINUNGSBILD
FARBLOSE BIS GELBE FLüSSIGKEIT MIT CHARAKTERISTISCHEM GERUCH.
CHEMISCHE GEFAHREN
Zersetzung beim Erhitzen oder Verbrennen unter Bildung giftiger Rauche mit Stickstoffoxiden. Reagiert sehr heftig mit Oxidationsmitteln, Nitraten und halogenierten Kohlenwasserstoffen. Greift einige Kunststoffe und Gummi an.
ARBEITSPLATZGRENZWERTE
TLV: 10 ppm (als TWA); Hautresorption; Krebskategorie A4 (nicht klassifizierbar als krebserzeugend für den Menschen); BEI vorhanden; (ACGIH 2005).
MAK: 5 ppm, 15 mg/m? Spitzenbegrenzung: überschreitungsfaktor II(4); Hautresorption; Schwangerschaft: Gruppe B; (DFG 2005).
AUFNAHMEWEGE
Aufnahme in den Körper durch Inhalation und über die Haut.
INHALATIONSGEFAHREN
Beim Verdampfen bei 20°C tritt langsam eine gesundheitsschädliche Kontamination der Luft ein.
WIRKUNGEN BEI KURZZEITEXPOSITION
WIRKUNGEN BEI KURZZEITEXPOSITION: Die Substanz reizt die Augen. Möglich sind Auswirkungen auf die Leber mit nachfolgender Gelbsucht (s. Anm.).
WIRKUNGEN NACH WIEDERHOLTER ODER LANGZEITEXPOSITION
Möglich sind Auswirkungen auf die Leber mit nachfolgenden Funktionsstörungen. Tierversuche zeigen, dass die Substanz möglicherweise fruchtbarkeitsschädigend oder entwicklungsschädigend wirken kann.
LECKAGE
Belüftung. Zündquellen entfernen. Ausgelaufene Flüssigkeit möglichst in abdichtbaren Behältern sammeln. Reste mit Sand oder inertem Absorptionsmittel aufnehmen und an einen sicheren Ort bringen. Persönliche Schutzausrüstung: Vollschutzanzug mit umgebungsluftunabhängigem Atemschutzgerät.
R-Sätze Betriebsanweisung:
R61:Kann das Kind im Mutterleib schädigen.
R20/21:Gesundheitsschädlich beim Einatmen und bei Berührung mit der Haut.
R36:Reizt die Augen.
S-Sätze Betriebsanweisung:
S53:Exposition vermeiden - vor Gebrauch besondere Anweisungen einholen.
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn möglich, dieses Etikett vorzeigen).
Aussehen Eigenschaften
C3H7NO; (Formyldimethylamin, N,N-Dimethylmethanamid, Ameisensäure-dimethylamid, DMF, DMFA). Farblose Flüssigkeit mit fischartig stechendem, auch ammoniak-ähnlichem Geruch.
Gefahren für Mensch und Umwelt
Heftige Reaktionen sind mit starken Oxidationsmitteln und anorganischen Nitraten möglich. Über stürmische Reaktionen mit Tetrachlorkohlenstoff und Hexachlorcyclohexan wird berichtet.
Die Dämpfe sind viel schwerer als Luft bildem bei höherer Temperatur mit Luft explosionsfähige Gemische.
Dimethylformamid ist gesundheitsschädlich beim Einatmen und bei Berührung mit der Haut. Es reizt die Augen. Nach oraler Aufnahme gastrointestinale Beschwerden. Der Stoff wird wegen seines universellen Lösevermögens leicht durch die Haut resorbiert.Symptome können sein: Appetitlosigkei, Übelkeit, Erbrechen, Sodbrennen, Magenschmerzen und kolikartige Leibschmerzen mit Durchfall.
Er kann ferner die Alkoholtoleranz erniedrigen und die Toxizität von gleicchzeitig einwirkenden Pharmaka oder Chemikalien erhöhen.
Bei Exposition Schwangerer kann eine Fruchtschädigung auch bei Einhaltung des MAK-Wertes nicht ausgeschlossen werden.
Schwach wassergefährdender Stoff (WGK 1).
Schutzmaßnahmen und Verhaltensregeln
Im Abzug arbeiten.
Schutzhandschuhe nur als kurzzeitiger Spritzschutz!
Verhalten im Gefahrfall
Mit flüssigkeitsbindendem Material z.B. Rench Rapid oder Chemizorb aufnehmen und als Sondermüll entsorgen.
Wasser, KohlendioxidPulver, Schaum.
Im Brandfall können nitrose Gase entstehen.
Erste Hilfe
Nach Hautkontakt: Bei Hautkontakt mit reichlich Wasser abwaschen.
Nach Augenkontakt: Bei geöffnetem Lidspalt mit reichlich Wasser mind. 10 Min. spülen. Augenarzt hinzuziehen.
Nach Einatmen: Sofort Frischluftzufuhr.
Nach Verschlucken: Sofort und wiederholt reichlich Wasser mit Zusatz von Aktivkohle und Natriumsulfat (1 Eßlöffel/1 Glas Wasser)trinken lassen. Arzt!
Nach Kleidungskontakt: Kontaminierte Kleidung sofort entfernen.
Ersthelfer: siehe gesonderten Anschlag
Sachgerechte Entsorgung
Als Sondermüll entsorgen.
Chemische Eigenschaften
N,N-Dimethylformamide is a colorless or slightly yellow liquid with a boiling point of 153°C and a vapor pressure of 380 Pa at 20°C. It is freely soluble in water and soluble in alcohols, acetone and benzene. N,N-Dimethylformamide is used as solvent, catalyst and gas absorbent. React violently with concentrated sulfuric acid, fuming nitric acid and can even explode. Pure Dimethylformamide is odorless, but industrial grade or modified Dimethylformamide has a fishy smell because it contains impurities of Dimethylamine. Dimethylformamide is unstable (especially at high temperatures) in the presence of a strong base such as sodium hydroxide or a strong acid such as hydrochloric acid or sulfuric acid, and is hydrolyzed to formic acid and dimethylamine.
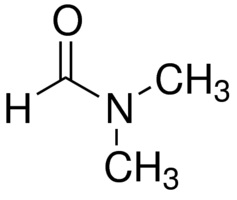
N,N-Dimethylformamide structure
Physikalische Eigenschaften
Clear, colorless to light yellow, hygroscopic, mobile liquid with a faint, characteristic, ammonialike
odor. An experimentally determined odor threshold concentration of 100 ppm
v was reported
by Leonardos et al. (1969).
Verwenden
N,N-Dimethylformamide (DMF) is a clear liquid that has been widely used in industries as a solvent, an additive, or an intermediate because of its extensive miscibility with water and most common organic solvents. Dimethylformamide solutions are used toprocess polymer fibers, films, and surface coatings; to permit easy spinning of acrylic fibers; to produce wire enamels, and as a crystallization medium in the pharmaceutical industry.
DMF can also be used for formylation with alkyllithium or Grignard reagents. It is used as a reagent in Bouveault aldehyde synthesis and also in Vilsmeier-Haack reaction. It acts as a catalyst in the synthesis of acyl chlorides. It is used for separating and refining crude from olefin gas. DMF along with methylene chloride acts as a remover of varnish or lacquers. It is also used in the manufacture of adhesives, fibers and films.
Application
The principal applications of N,N-dimethylformamide are as a solvent and as an extractant, particularly for salts and compounds with high molecular mass. This role is consistent with its interesting combination of physical and chemical properties: low molecular mass, high dielectric constant, electron-donor characteristics, and ability to form complexes. The use of DMF as a component in synthesis is of relatively minor significance, at least commercially. N,N-Dimethylformamide (anhydrous) has been used as solvent for the synthesis of cytotoxic luteinizing hormone-releasing hormone (LH-RH) conjugate AN-152 (a chemotherapeutic drug) and fluorophore C625 [4-(N,N-diphenylamino)-4′-(6-O-hemiglutarate)hexylsulfinyl stilbene]. It may be employed as solvent medium for the various organic reduction reactions.
Vorbereitung Methode
Industrial production of N,N-Dimethylformamide (DMF) is via three separate processes (Eberling 1980). Dimethylamine in methanol is reacted with carbon monoxide in the presence of sodium methoxide or metal carbonyls at 110-150°C and high pressure. Alternately, methyl formate is produced from carbon monoxide and methanol under high pressure at 60-100°C in the presence of sodium methoxide. The resulting methyl formate is distilled and then reacted with dimethylamine at 80-100°C and low pressure. The third process involves reaction of carbon dioxide, hydrogen and dimethylamine in the presence of halogen-containing transition metal compounds to yield DMF.
Definition
ChEBI: N,N-dimethylformamide is a member of the class of formamides that is formamide in which the amino hydrogens are replaced by methyl groups. It has a role as a polar aprotic solvent, a hepatotoxic agent and a geroprotector. It is a volatile organic compound and a member of formamides. It is functionally related to a formamide.
synthetische
Two processes are used commercially to produce dimethylformamide. In the direct or one-step process, dimethylamine and carbon monoxide react at 100°C and 200 psia in the presence of a sodium methoxide catalyst to make dimethylformamide. The homogenous catalyst is separated from the crude DMF, which is then refined to the final product. In the indirect process, methyl formate is isolated, and then reacted with dimethylamine to form DMF. To obtain methyl formate, two methods may be used - dehydrogenation of methanol and esterification of formic acid.
The two-step process for the synthesis of N,N-dimethylformamide differs from direct synthesis because methyl formate is prepared separately and introduced in the form of ca. 96% pure (commercialgrade) material. Equimolar amounts of methyl formate and N,N-dimethylamine are subjected to a continuous reaction at 60-100°C and 0.1 – 0.3 MPa. The resulting product is a mixture of N,N-dimethylformamide and methanol. The purification process involves distillation and is analogous to that described for direct synthesis. However, no separation of salts is required because no catalysts are involved in the process. According to the corrosive properties of both starting materials and products, stainless steel has to be used as material of construction for production facilities.
Allgemeine Beschreibung
A water-white liquid with a faint fishy odor. Flash point 136°F. Slightly less dense than water. Vapors heavier than air. Toxic by inhalation or skin absorption. May irritate eyes.
Air & Water Reaktionen
Flammable. Water soluble.
Reaktivität anzeigen
N,N-Dimethylformamide may react violently with a broad range of chemicals, e.g.: alkaline metals (sodium, potassium), azides, hydrides (sodium borohydride, lithium aluminum hydride), bromine, chlorine, carbon tetrachloride, hexachlorocyclohexane, phosphorus pentaoxide, triethylaluminum, magnesium nitrate, organic nitrates. Forms explosive mixtures with lithium azide [Bretherick, 5th ed., 1995, p. 453]. Oxidation by chromium trioxide or potassium permanganate may lead to explosion [Pal B. C. et al., Chem. Eng. News, 1981, 59, p. 47].
Health Hazard
The acute toxicity of DMF is low by inhalation, ingestion, and skin contact. Contact
with liquid DMF may cause eye and skin irritation. DMF is an excellent solvent for
many toxic materials that are not ordinarily absorbed and can increase the hazard of
these substances by skin contact. Exposure to high concentrations of DMF may lead
to liver damage and other systemic effects.
Dimethylformamide is listed by IARC in Group 2B ("possible human carcinogen").
It is not classified as a "select carcinogen" according to the criteria of the OSHA
Laboratory Standard. No significant reproductive effects have been observed in
animal tests. Repeated exposure to DMF may result in damage to the liver, kidneys,
and cardiovascular system
Flammability and Explosibility
DMF is a combustible liquid (NFPA rating = 2). Vapors are heavier than air and may travel to source of ignition and flash back. DMF vapor forms explosive mixtures with air at concentrations of 2.2 to 15.2% (by volume). Carbon dioxide or dry chemical extinguishers should be used to fight DMF fires.
Industrielle Verwendung
World production capacity of DMF is about 225 x 10
3 tons per year. The main application of DMF is as solvent in industrial processes, especially for polar polymers such as Polyvinylchloride, polyacrylonitrile and polyurethanes. DMF solutions of high molecular weight polymers are processed to fibers, films, surface coatings and synthetic leathers. Since salts can be dissolved and dissociated in DMF, the solutions are used in electrolytic capacitors and certain electrolytic processes (Eberling 1980).
Kontakt-Allergie
This is an organic solvent for vinyl resins and acetylene, butadiene, and acid gases. It caused contact dermatitis in a technician at an epoxy resin factory and can provoke alcohol-induced flushing in exposed subjects.
Carcinogenicity
DMF is not carcinogenic to
animals except under very high inhalation exposure conditions.
No increase in tumors was seen in rats that
inhaled 25, 100, or 400 ppm for 6 h/day, 5 days/week for
2 years. Similarly, no tumors were produced
in mice under the same conditions for 18 months. In
that chronic experiment, rats and mice were exposed by
inhalation (6 h/day, 5 days/week) to 0, 25, 100, or 400 ppm
DMF for 18 months (mice) or 2 years (rats). Body weights
of rats exposed to 100 (males only) and 400 ppm were
reduced and, conversely, body weights were increased in
400 ppm mice. Serum sorbitol dehydrogenase activity was
increased in rats exposed to 100 or 400 ppm. DMF-related
morphological changes in rats were observed only in the
liver and consisted of increased relative liver weights,
centrilobular hepatocellular hypertrophy, lipofuscin/hemosiderin
accumulation in Kupffer cells, and centrilobular
single cell necrosis (400 ppm only). The same liver effects
were seen in all groups of mice, although the response at
25 ppm was judged as minimal.
Environmental Fate
Biological. Incubation of [
14C]N,N-dimethylformamide (0.1–100 μg/L) in natural seawater
resulted in the compound mineralizing to carbon dioxide. The rate of carbon dioxide formation
was inversely proportional to the initial concentration (Ursin, 1985).
Chemical. Reacts with acids or bases forming formic acid and dimethylamine (BASF, 1999)
Stoffwechselwegen
Three urinary metabolites are identified in humans and
rodents, and the metabolites quantified are N-
(hydroxymethyl)-N-methylformamide (HMMF), resulting
in N-methylformamide (NMF) and N-acetyl-S-(N-
methylcarbamoyl)cysteine (AMCC). Ten volunteers
who absorb between 28 and 60 mmol/kg DMF during
an 8 h exposure to DMF in air at 6 mg=m3 excrete in
the urine within 72 h between 16.1 and 48.7% of the
dose as HMMF, between 8.3 and 23.9% as
formamide, and between 9.7 and 22.8% as AMCC.
AMCC together with HMMF is also detected in the
urine of workers after occupational exposure to DMF.
There is a quantitative difference between the
metabolic pathway of DMF to AMCC in humans and
rodents.
Lager
DMF should be used only in areas free of ignition sources, and quantities greater than 1 liter should be stored in tightly sealed metal containers in areas separate from oxidizers.
Inkompatibilitäten
Though stable at normal temperatures and storage conditions, DMF may react violently with halogens, acyl halides, strong oxidizers, and polyhalogenated compounds in the presence of iron. Decomposition products include toxic gases and vapors such as dimethylamine and carbon monoxide. DMF will attack some forms of plastics, rubber, and coatings.
Waste disposal
Excess DMF and waste material containing this substance should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines.
N,N-Dimethylformamid Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
EMITEFUR
Indole-5-carboxaldehyde
Halofuginone
ETHYL 2,4-DIMETHYLQUINOLINE-3-CARBOXYLATE
2,2'-BITHIOPHENE-5-CARBOXALDEHYDE
4-BENZYLOXY-2-NITROTOLUENE
3-CHLORO-2-HYDROXY-5-NITROPYRIDINE
Pyridine-2-carbonyl chloride hydrochloride
1-(3-Pyridyl)-3-(dimethylamino)-2-propen-1-one
Bifendate
Olprinone
Methylprednisolone aceponate
4-METHYL-2-(1H-PYRROL-2-YL)QUINOLINE
1-[(4-METHYLPHENYL)SULFONYL]-1H-INDOLE-3-CARBALDEHYDE
1-METHYL-1H-PYRAZOLO[3,4-B]PYRIDIN-3-YLAMINE
4-Acetamido-3-nitrobenzoesure
9-DIETHYLAMINO-2-HYDROXY-5H-BENZ(A)-
5-Methylpyridin-2-carbonitril
Etomidat
a new kind of liquid crystal copolymer
ETHYL 2-(CHLOROMETHYL)-4-PHENYLQUINOLINE-3-CARBOXYLATE
3,5-Difluorophenylacetic acid
ETHYL 2-(CHLOROMETHYL)-4-PHENYLQUINOLINE-3-CARBOXYLATE HYDROCHLORIDE
1-Ethylpyrrolidin-2-ylmethylamin
1-[2,6-DICHLORO-4-(TRIFLUOROMETHYL)PHENYL]-2,5-DIMETHYL-1H-PYRROLE-3-CARBALDEHYDE
Benzo-1,3-dioxol-4-carboxaldehyd
4-Amino-6-chlorpyrimidin-5-carbaldehyd
N-METHYL-2-PIPERAZIN-1-YLACETAMIDE
Octenidine
4-(1H-IMIDAZOL-1-YL)BENZOIC ACID
1-(1-Methyl-4-piperidinyl)piperazine
2-Chlor-4-(dimethylamino)pyrimidin
1-(2,4-DIMETHYLQUINOLIN-3-YL)ETHANONE HYDROCHLORIDE
(2-METHYL-4-PHENYLQUINOLIN-3-YL)ACETIC ACID HYDROCHLORIDE
Labetalol
THIOPHENE-3,4-DICARBOXYLIC ACID
RARECHEM AL BI 1318
Cefpimizole
4,6-DIMETHOXYPYRIMIDINE-2-CARBONYL CHLORIDE
4,6-Dichloro-5-pyrimidinecarbaldehyde

